Intraoperative Functional Mapping
George A. Ojemann
Susan Y. Bookheimer
Introduction
This chapter describes established intraoperative methods for identifying functionally important cortex during resective epilepsy surgery. Several other chapters are complementary. A promising new technique for intraoperative functional localization, optical imaging of the “intrinsic signal,” is discussed in Chapter 86. Like extraoperative methods for functional localization discussed in Chapter 175, the established intraoperative methods rely on electrical stimulation mapping. Although the technical details of stimulation mapping differ in the different settings, several controversial issues concern both methods. How reliable is stimulation mapping in identifying areas that must be spared in a resection to avoid a functional deficit? When is stimulation mapping required for planning a cortical resection? Compared to intraoperative methods, extraoperative methods expose the patient to additional risks (including an extra craniotomy) and require a substantial additional investment of health care resources.23 An additional area of controversy, then, is when the additional risk and cost of the extraoperative method are justified.
Application of an electric current to the cortical surface has a variety of effects, exciting both neurons and en passage fibers and also blocking their function, effects that can produce excitation and inhibition locally or at a distance.37 Thus, the effects of stimulation cannot be easily predicted physiologically but rather must be determined empirically. In the quiet patient, responses are readily evoked from primary motor and somatosensory cortex (localized movements or dysesthesias), somewhat more rarely from primary visual cortex (localized phosphenes), and quite infrequently from primary auditory cortex. There are usually no responses from stimulation of other cortical areas at currents below the threshold for afterdischarges, although in patients with temporal lobe epilepsy, larger currents associated with afterdischarge will occasionally evoke the interpretive and experiential responses studied by Penfield et al.8,20,34 However, if the patient engages in an ongoing measure of language, stimulation of some dominant hemisphere cortical areas outside primary cortices will disrupt language performance. Presumably the predominant effect of stimulation at those sites is a disruption of function, probably by depolarization blockade. This is the technique of intraoperative stimulation mapping of language developed initially by Penfield and Roberts.35
The choice of language measure to use with stimulation mapping is somewhat controversial. Penfield used object naming. This also has the advantage as a screening measure for language function that all aphasic syndromes include deficits in naming. This is the language measure most often used by the author.31 However, reading has also been used as a screening test, particularly with extraoperative stimulation.18
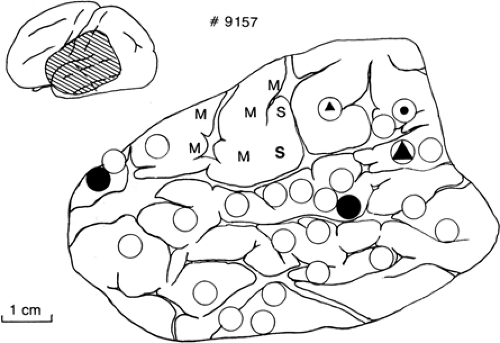
The reliability of stimulation as a technique for identifying functionally important areas depends on how localized the effects are and how reliably those effects predict the effect of resecting that cortex. Intraoperative imaging of the changes in the “intrinsic signal” indicating where the stimulating current is altering neurons has shown that with bipolar cortical surface stimulation below the threshold for afterdischarge, as used by the author, the changes are confined to tissue between the electrodes in both man11 and animals.10 Behaviorally, both sensory-motor and language effects of intraoperative stimulation are usually localized on a scale of millimeters to a few centimeters. Threshold sensory-motor effects with intraoperative cortical stimulation are usually confined to a few millimeters on each side of the central sulcus, showing the classical homuncular pattern of localization. Sensory-motor changes with extraoperative stimulation are often evoked from a wider area.17 Sites where intraoperative stimulation repeatedly evokes naming errors are often confined to several separate cortical sites, each 1 to 2 cm2 in extent, often with sharp boundaries (Fig. 1).31 Extraoperative stimulation mapping of language has often shown changes from somewhat wider areas.16,32
Evidence from several studies has shown that perisylvian sites identified as important to naming by intraoperative stimulation mapping can predict the language effects of a resection. In resections for epilepsy, Ojemann and Dodrill27 found that when anterior temporal resections came within 2 cm along a continuous gyrus of a site where stimulation evoked repeated object-naming errors, testing with a sensitive aphasia battery 1 month after operation showed subtle language changes that were not present when the resection had not come within 2 cm and that were not related to the size of the resection, the degree of seizure control, or the patient’s preoperative verbal abilities. In temporal lobe tumor resections, Haglund et al.9 found that when the resection came within 5 mm of a site showing repeated naming errors with intraoperative stimulation, about one third of patients had a permanent postoperative clinically evident aphasia, whereas when these sites were 15 mm or more from the margin of the resection, no patient had even a temporary clinically evident aphasia. Very large resections in classical language cortex have not been followed by postoperative aphasias when stimulation mapping demonstrated all essential sites for naming elsewhere.31 Thus, stimulation and lesions seem to identify the same cortical sites as essential to language. Interestingly, surface cortical stimulation predicts effects of resections that include both surface and buried cortex, suggesting that essential language areas are rarely if ever located only in buried cortex.
On the other hand, stimulation in the dominant hemisphere outside of the perisylvian area evokes repeated changes in language measures in areas where resection does not lead to a permanent language deficit. These include the supplementary motor area in the superior frontal lobe,7,24,35 where resection of sites with repeated naming or reading errors often results
in a dramatic initial deficit (patients may be mute), but with rapid resolution, so that there is little or no permanent language change.39 Similarly, repeated language errors have been evoked by stimulation of basal temporal cortex,18 but resection of this area rarely is associated with a persisting language deficit.
in a dramatic initial deficit (patients may be mute), but with rapid resolution, so that there is little or no permanent language change.39 Similarly, repeated language errors have been evoked by stimulation of basal temporal cortex,18 but resection of this area rarely is associated with a persisting language deficit.
Indications
The location of eloquent areas is mainly a concern in planning cortical resections in central portions of the hemisphere and, in the language-dominant hemisphere, posterior frontal and posterior temporal-inferior parietal areas. Although language is usually lateralized to the left hemisphere, statistically independent of handedness,48 when there is any question about unusual language lateralization, this should be established preoperatively with the intracarotid amobarbital perfusion test.46 Eloquent areas can be identified either anatomically or functionally. However, the anatomic landmarks that identify rolandic cortex, vertically oriented gyri with a “U” shape at the sylvian fissure, are often hard to discern through the intact pia and may be displaced by tumors. Common anatomic landmarks for avoiding language areas in the dominant hemisphere are anterior to the pterion for frontal resections and, for temporal resections, the line of rolandic cortex or the vein of Labbe or 4 to 4.5 cm from the temporal tip, sparing superior gyrus.
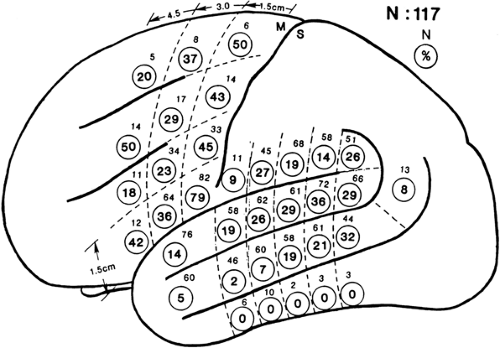
Electrical stimulation mapping often localizes language to several quite focal perisylvian areas in an individual patient, but these sites are in somewhat different places in different patients (Fig. 2).31 This variability in exact location of language areas is such that only the most posterior portion of inferior frontal gyrus, immediately in front of face motor cortex, is essential for language in a large proportion of patients. Elsewhere, including any portion of the entire Wernicke area, essential language areas are present in not much more than a third of patients. This substantial variability is one of the strongest arguments for mapping function in each patient rather than depending on anatomic landmarks derived from population studies. Use of anatomic landmarks rather than individual stimulation mapping to localize language unnecessarily restricts resections for many patients while not avoiding essential language areas in a few patients.31 Thus, stimulation mapping is of value in safely maximizing resections in posterior frontal and posterior temporal lobes, whether the resection is for epilepsy or for a structural lesion including tumors and vascular malformations.2
Whether location of language areas is important to planning anterior temporal resections for epilepsy in the dominant hemisphere is controversial.32 Stimulation mapping has occasionally identified sites related to language within 3 cm of the temporal tip, even in middle temporal gyrus. There are no randomized studies to resolve this issue, but a report of consecutive series of anterior temporal lobectomies with or without identification of language areas suggested that there was a slightly greater risk of a postoperative language deficit in the series done without identification of language areas, although the difference was small.1,13
Technique1
Motor cortex can be identified under either general or local anesthesia with intraoperative stimulation mapping, although motor mapping under general anesthesia requires a technique in which the patient is not paralyzed, and the resulting motor map is usually rather crude, with responses only at large currents; the extensive area devoted to tongue movement usually cannot be identified. An alternative method for intraoperative identification of rolandic cortex under general anesthesia is recording of somatosensory-evoked potentials (SSEPs).47 However, with this technique, too, identification of face and tongue representation is difficult, and the author finds SSEP recording more time consuming than stimulation mapping. Detailed intraoperative mapping of rolandic cortex and intraoperative mapping of language require that the patient be awake, under local anesthesia, for that portion of the operation. Having the patient awake for part of the operation also allows recording of the electrocorticogram (ECoG) without alterations induced by general anesthetic agents. Thus, we discuss the technique of awake craniotomy with modern intravenous propofol anesthesia, followed by the technique of intraoperative stimulation mapping.
The advent of propofol intravenous anesthesia has made “awake” craniotomy much easier for both the patient and surgeon.42 About the only demand now made on the patient is that he or she be able to hold still for 1 to 2 hours while awake. Thus, the technique can easily be used in children aged 12 or older and most adolescents and adults. It can also be safely used in the presence of an intracranial mass, although the brain will
be slightly “tighter” than is usually observed with modern endotracheal anesthesia, and it may be necessary to be slightly more aggressive with the use of intravenous osmotic agents in that setting compared to general anesthesia.
be slightly “tighter” than is usually observed with modern endotracheal anesthesia, and it may be necessary to be slightly more aggressive with the use of intravenous osmotic agents in that setting compared to general anesthesia.
We now use only the lateral position with propofol. With that position we have not had difficulties maintaining an airway; those problems have occurred in patients in the supine position. The patient is positioned while awake, with particular attention to his or her comfort. The head rests on a foam “doughnut”; skeletal fixation is not used. Propofol anesthesia is then induced intravenously. Once the patient is asleep, a local anesthetic field block is placed, using a mixture of equal volumes of 0.5% lidocaine and 0.25% bupivacaine (Marcaine), both with 1:200,000 epinephrine. Propofol is not a particularly good analgesic, so the “asleep” patient will often show more reaction to the placement of this block than will an awake patient. Thus, we use the same technique, making the first injections slowly through a 30-gauge needle at sites near the major scalp nerves. Use of small amounts of intravenous fentanyl is of value in reducing responses to placement of the block. If the incision is to extend to the root of the zygoma, the insertions of the temporalis muscle are also infiltrated. The scalp incision and craniotomy then proceed in the usual manner. Once the dura is exposed, dural pain sensation is blocked by intradural injection of small quantities of the local anesthetic on each side of the middle meningeal artery, using the 30-gauge needle. A clamp is placed on the skull at the edge of the craniotomy, not only to provide a place to attach ECoG recording equipment, but also to provide a handle to control the head if the patient becomes restless.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree