Figure 53.1. Intraventricular hemorrhage in a 45-year-old man due to a left caudate hemorrhage extending caudally to the fourth ventricle.
Predominance of blood in the fourth ventricle with associated subarachnoid hemorrhage in the basal cisterns is typical for ruptured aneurysms of the posterior inferior cerebellar artery. Isolated IVH in young adults should raise the suspicion for an arteriovenous malformation (AVM). IVH in association with medial thalamic or caudate intraparenchymal hemorrhage is common for patients with hypertensive ICH. In cases where the etiology cannot be delineated from CT scan alone, a gadolinium enhanced magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) of the brain is indicated to further explore and seek the etiology (Figure 53.2).
Angiography is reserved for cases with a high suspicion for AVM or aneurysms.

Figure 53.2. Intraventricular hemorrhage in a 34-year-old man without evidence of a pre-existing ICH and a normal cerebral angiogram.
53.4 Pathophysiology of IVH
Acute IVH leads to acute intracranial hypertension and obstructive hydrocephalus, which then exerts pressure onto the ascending reticular activating system (ARAS) resulting in a depressed level of arousal. The presence of blood inside the ventricles may lead to obstructive hydrocephalus and subsequently to elevated intracranial pressure (ICP). Elevated ICP may significantly lower cerebral perfusion pressure (CPP) leading to cerebral ischemia, further augmenting brain injury [10].
Some studies support the theory that the blood itself causes damage to the ependymal and subependymal layers of the brain [11]. Neuropsychological data has shown that patients with IVH suffer from significant cognitive deficits even after the blood has completely disappeared, suggesting that the harmful effects of intraventricular blood may not only be due to the mere presence of blood in the ventricles, but a more complex neuro-hormonal mechanism [12]. It is likely that subarachnoid fibrosis, extensive ependymal cell loss, and subependymal glial proliferation on the walls of the ventricles is produced by blood in the cerebrospinal fluid, most likely as an inflammatory response [11,13-15].
There is very little known about the fibrinolytic system in the cerebrospinal fluid and brain tissue. Activity is localized in the vascular endothelial cells, meninges and choroid plexus, with minimal amounts of tissue-type plasminogen activator (t-PA) in the CSF of adults with no neurologic disease. In the event of ICH, increased levels of t-PA have been observed in the CSF. It is not known whether the increased levels result from an enhancement of local production or from leakage across a damaged blood-brain barrier [16,17]. Interestingly, this increase in fibrinolytic activity after an intracranial hemorrhage does not result in a rapid resolution of intraventricular blood [16-18]. Studies of serial CT scans performed on patients with IVH have seemed to suggest that the intraventricular blood gradually disappears over a period of 2 to 3 weeks after the hemorrhage [19,20]. In contrast, post-mortem studies have revealed the persistence of intraventricular blood for months after a hemorrhage [21].
53.5 Clinical Management of IVH
Treatment of IVH begins with stabilization of the patient followed by admission to an intensive care unit (ICU) where airway, neurological and hemodynamic surveillance and support are more meticulously possible. An official consensus or guideline on all aspects of IVH management – blood pressure control, indications for placing a ventricular drain, and administration of fibrinolytics – does not exist. However, lower mortality rates have been consistently observed when the treatment policy involves: 1) the use of an intraventricular drainage catheter (ventriculostomy drain) to maintain ICP within the normal range (usually less than 15-20 mmHg), and 2) Optimal management of blood pressure [5]. Management of IVH is multi-faceted and solely maintaining ICP within a normal range is insufficient to impact mortality or morbidity [22].
In our practice, all patients with IVH are admitted to the ICU for close monitoring of neurological status and hemodynamics. An intraventricular catheter is placed for emergent ICP control in patients with poor neurologic status (usually with Glasgow Coma Scale of 8 or less, signs of impending herniation) and/or radiographic evidence of ventriculomegaly secondary to the IVH. This catheter is typically kept open for continuous cerebrospinal fluid (CSF) drainage. It is crucial to correct any coagulopathies that may have contributed to the initial hemorrhage and prior to placement of this catheter. For control of elevated blood pressure, we follow the most recent American Stroke Association guidelines for management of spontaneous ICH and aneurysmal SAH: a target systolic value of 160 mmHg or less [23,24]. Anticonvulsant medications are not routinely used for isolated IVH, unless seizures were a part of the initial clinical presentation. Administration of intravenous recombinant activated factor VIIa (rFVIIa) has been shown to reduce the expansion of ICH volume when administered within 4 hours of symptom onset, but without a significant impact on survival or functional outcome [25]. There is some evidence that in patients with IVH, rFVIIa treatment may contribute to development of hydrocephalus, perhaps due to its pro-coagulant effect leading to delayed resolution of intraventricular blood [26].
A theory exists that accelerated clearance of the intraventricular clot using direct injection of thrombolytics such as recombinant tissue-type plasminogen activator (rt-PA) into the ventricles may have a positive impact on clinical outcome. The safety and efficacy of this treatment in increasing the rate of clot lysis has been demonstrated [27]. Intraventricular thrombolysis does appear to reduce mortality rate and accelerate blood clot resolution in patients with moderate to severe IVH [28,29]. Furthermore, functional outcome measures (Glasgow outcome scale, modified Rankin scale and National Institutes of Health Stroke Scale) seem to be improved in patients treated with rt-PA despite a trend toward more bleeding complications. Questions remain as to whether rt-PA is the right thrombolytic agent and what the appropriate dose is. This treatment is currently being studied in a phase III clinical (Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III: CLEAR III). In some centers, intraventricular rt-PA is used in an “off label” fashion. We have successfully used small intraventricular doses of 1 to 2 mg rt-PA to re-establish the patency of a ventricular drain that has been obstructed by blood. For intraventricular fibrinolytic therapy, doses ranging from 2 to 4 mg of rt-PA at 12 to 24 hour intervals can be safely administered with minimal risk of bleeding complications. The injection of the fibrinolytic agent should be preceded by withdrawal of a volume of CSF equivalent to the drug volume being injected, to avoid a sudden increase in intracranial pressure. Injection is followed by a preservative free normal saline flush of 1 to 2 ml and clamping of the ventricular drain for 30 minutes to an hour. The ICP is closely monitored during this time period and if the pressures rise above 20 mmHg sustained over 5 minutes the ventricular drain is reopened, allowed to drain 5 to 10 ml and then closed again. If ICP continues to be elevated the drain is just left open and t-PA injected again in 12 to 24 hours if necessary. The decision to treat again is based on clinical status and residual intraventricular blood on follow up imaging studies
Prior to even considering fibrinolytics for the treatment of IVH the physician must be certain that the acute episode of bleeding has subsided and that any underlying condition contributing to the hemorrhage (hypertension, coagulopathy, aneurysm) has been corrected. This is critical in the management, as the administration of intraventricular fibrinolytics to a patient with vascular pathology (unsecured aneurysm) could be fatal. A repeat CT scan of the brain is performed within 12-24 hours of the onset of bleeding to assess stability of the hemorrhage.
Many practical issues concerning intraventricular fibrinolytic therapy still need to be addressed. The criteria for patient selection and the determination of an appropriate dose, timing, and protocol for administration are all the focus of CLEAR III.
53.6 Prognosis
The morbidity and mortality of patients with IVH largely depends on the extent and the volume of blood in the ventricular system [4,7,30,31]. Patients with secondary IVH fare more poorly than those with primary IVH [31,32]. If an ICH ruptures into the ventricular system, the mortality increases from 30% to 44% and if it involves all four ventricles, it can be as high as 60 to 91% [31,32]. As for the location, thalamic ICH patients with intraventricular extension seem to have the worst prognosis. Additional predictors of outcome include initial Glasgow Coma Scale and age greater than 80 years [4,31,32]. Long term consequence of IVH may involve a severe, persisting amnesic state [33].
53.7 Conclusions
IVH is an entity that can be associated with a large number of causes. The clinical picture depends on the severity of the hemorrhage and management consists of identifying the underlying etiology and placing an external ventricular drain to control and monitor intracranial pressure. Although reduced mortality rates have been reported with intraventricular fibrinolytic therapy, it is not clearly known if it improves neurologic outcomes.
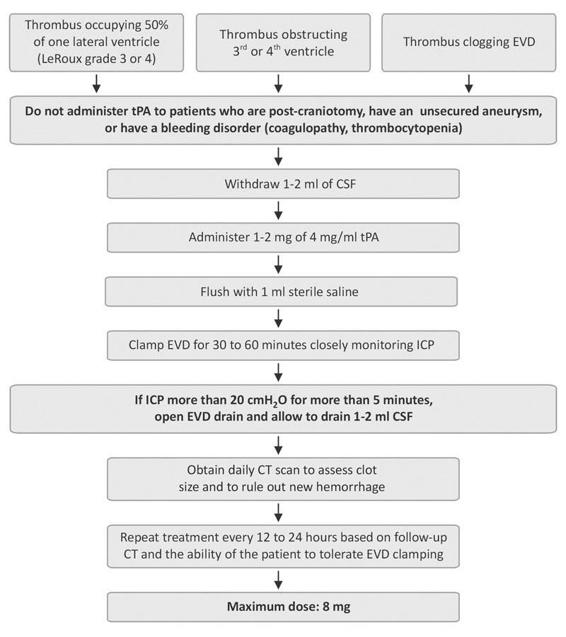
Figure 53.3. Example protocol for intraventricular thrombolysis.
References
1. Hallevi H, Albright KC, Aronowski J, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology 2008; 70: 848-52
2. Bhattathiri PS, Gregson B, Prasad KS, et al. Intraventricular hemorrhage and hydrocephalus after spontaneous intracerebral hemorrhage: results from the STICH trial. Acta Neurochir (Suppl) 2006; 96: 65-8
3. Steiner T, Diringer MN, Schneider D, et al. Dynamics of intraventricular hemorrhage in patients with spontaneous intracerebral hemorrhage: risk factors, clinical impact, and effect of hemostatic therapy with recombinant activated factor VII. Neurosurgery 2006; 59: 767-73; discussion 73-4
4. Hemphill JC 3rd, Bonovich DC, Besmertis L, et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001; 32: 891-7
5. Hanley DF. Intraventricular hemorrhage: severity factor and treatment target in spontaneous intracerebral hemorrhage. Stroke 2009; 40: 1533-8
6. Engelhard HH, Andrews CO, Slavin KV, et al. Current management of intraventricular hemorrhage. Surg Neurol 2003; 60: 15-21; discussion -2
7. Graeb DA, Robertson WD, Lapointe JS, et al. Computed tomographic diagnosis of intraventricular hemorrhage. Etiology and prognosis. Radiology 1982; 143: 91-6
8. LeRoux PD, Haglund MM, Newell DW, et al. Intraventricular hemorrhage in blunt head trauma: an analysis of 43 cases. Neurosurgery 1992; 31: 678-84; discussion 84-5
9. Hallevi H, Dar NS, Barreto AD, et al. The IVH score: a novel tool for estimating intraventricular hemorrhage volume: clinical and research implications. Crit Care Med 2009; 37: 969-74
10. Mayer SA, Thomas CE, Diamond BE. Asymmetry of intracranial hemodynamics as an indicator of mass effect in acute intracerebral hemorrhage. A transcranial Doppler study. Stroke 1996; 27: 1788-92
11. Andrews CO, Engelhard HH. Fibrinolytic therapy in intraventricular hemorrhage. Ann Pharmacother 2001; 35: 1435-48
12. Hutter BO, Kreitschmann-Andermahr I, Gilsbach JM. Cognitive deficits in the acute stage after subarachnoid hemorrhage. Neurosurgery 1998; 43: 1054-65
13. Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery 1986; 19: 553-72
14. Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery 1986; 19: 547-52
15. Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1. Canine intraventricular blood cast model. Neurosurgery 1986; 19: 540-6
16. Whitelaw A, Creighton L, Gaffney P. Fibrinolysis in cerebrospinal fluid after intraventricular haemorrhage. Arch Dis Child 1991; 66: 808-9
17. Wu KK, Jacobsen CD, Hoak JC. Plasminogen in normal and abnormal human cerebrospinal fluid. Arch Neurol 1973; 28: 64-6
18. Whitelaw A, Saliba E, Fellman V, et al. Phase I study of intraventricular recombinant tissue plasminogen activator for treatment of posthaemorrhagic hydrocephalus. Arch Dis Child Fetal Neonatal Ed 1996; 75: F20-26
19. Bakshi R, Kamran S, Kinkel PR, et al. MRI in cerebral intraventricular hemorrhage: analysis of 50 consecutive cases. Neuroradiology 1999; 41: 401-9
20. Bakshi R, Kamran S, Kinkel PR, et al. Fluid-attenuated inversion-recovery MR imaging in acute and subacute cerebral intraventricular hemorrhage. AJNR Am J Neuroradiol 1999; 20: 629-36
21. Yamamoto Y, Waga S. Persistent intraventricular hematoma following ruptured aneurysm. Surg Neurol 1982; 17: 301-3
22. Nieuwkamp DJ, de Gans K, Rinkel GJ, et al. Treatment and outcome of severe intraventricular extension in patients with subarachnoid or intracerebral hemorrhage: a systematic review of the literature. J Neurol 2000; 247: 117-21
23. Morgenstern LB, Hemphill III JC, Anderson C, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage. Stroke 2010; 41: 2108-29
24. Connolly Jr ES, Rabinstein AA, Carhuapoma JR, et al. Guidelines for the Management of Aneurysmal Subarachnoid Hemorrhage. Stroke 2012; 43: 1711-37
25. Mayer SA, Brun NC, Begtrup K, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008; 358: 2127-37
26. Subramanian S, Denchuk AM, Watson T, Barber PA, Hill MD. Unexpected posthemorrhagic hydrocephalus in patients treated with rFVIIa. Neurology 2007; 68: 1084
27. Morgan T, Awad I, Keyl P, et al. Preliminary report of the clot lysis evaluating accelerated resolution of intraventricular hemorrhage (CLEAR-IVH) clinical trial. Acta Neurochir Suppl 2008; 105: 217-20
28. Coplin WM, Vinas FC, Agris JM, et al. A cohort study of the safety and feasibility of intraventricular urokinase for nonaneurysmal spontaneous intraventricular hemorrhage. Stroke 1998; 29: 1573-9
29. Naff NJ, Williams MA, Keyl PM, et al. Low-dose recombinant tissue-type plasminogen activator enhances clot resolution in brain hemorrhage. The intraventricular hemorrhage thrombolysis trial. Stroke 2011; 42: 3009-16
30. Shapiro SA, Campbell RL, Scully T. Hemorrhagic dilation of the fourth ventricle: an ominous predictor. J Neurosurg 1994; 80: 805-9
31. Tuhrim S, Horowitz DR, Sacher M, et al. Volume of ventricular blood is an important determinant of outcome in supratentorial intracerebral hemorrhage. Crit Care Med 1999; 27: 617-21
32. Young WB, Lee KP, Pessin MS, et al. Prognostic significance of ventricular blood in supratentorial hemorrhage: a volumetric study. Neurology 1990; 40: 616-9
33. Darby DG, Donnan GA, Saling MA, et al. Primary intraventricular hemorrhage: clinical and neuropsychological findings in a prospective stroke series. Neurology 1988; 38: 68-75
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






