Fig. 1.1
Depiction of the three therapeutic approaches to acute brain injury. (Note that devices also have potential from clot-retrieval devices to devices that stimulate the sphenopalatine ganglia to blood pressure cuffs that deliver remote limb ischemic conditioning)
While there remains “hope” that cell therapies will be effective in stroke and brain injury there is an also an excess of “hype.” The term “stem cells” often incites unrealistic expectations among our patients, their caregivers and families, and the press. Direct-to-consumer (DTC) marketing in this digital age with social media permits easier exploitation of patients and families [11]. Patient desperation and hyping of stem cells has led to “stem cell tourism” where patients and families often travel overseas for expensive and unproven treatments with cost estimates of US$ 47,000 per treatment. In the USA, the FDA regulates cell therapies if they are “nonhomologous” and the cells are more than “minimally manipulated.” “Homologous” function means that the stem cell has the same function in the donor and recipient. Hence, bone marrow cells for bone marrow transplant in a recipient with bone marrow failure are not regulated; however if bone marrow cells are used to repair the brain, then they are “nonhomologous” and under the purview of the FDA.
From a societal standpoint, only the oversight of regulatory agencies such as the FDA with the support of our professional societies will protect our patients and their families from modern “snake oil salesmen” and unscrupulous operators. Simultaneously, we need to uphold the highest standards for preclinical work and clinical trials. Only rigorous preclinical testing and randomized, blinded clinical trials will be the antidote to the hype of stem cell therapies and stem cell tourism. Due to the many failed clinical trials in stroke and other neurological diseases, there are now calls for more rigorous methodology and transparency in reporting of results of preclinical testing [12]. This includes randomization, concealment of allocation, blinding, sample size estimation, and reporting of negative studies. Organizations of clinicians and representatives from academia, industry, the National Institutes of Health (NIH), and the FDA such as The Stroke Academic Industry RoundTable (STAIR) and Stem Cells as an Emerging Paradigm for Stroke (STEPS) have published criteria to follow to help ensure that the best therapies are brought forward into clinical trial and that the clinical trials are conducted with the optimal design [13–17].
Types of Cells and Timing
With reparative and restorative cell therapies, a variety of types of cells, routes of administration, and “timing” of administration are proposed. The optimal timing and optimal routes of administration are not precisely known but likely will be related to the cell type used. The “golden time” of cell-based therapeutics is likely in the first week or weeks after injury when the brain is actively remodeling (Fig. 1.2). Intravascular (intravenous (IV) and intra-arterial (IA)) administration routes will likely be used in this time period. This early time point presents some challenges. There may not allow sufficient time to isolate and expand certain types of autologous stem cells and allow them to be transplanted in a short time window. In addition, the stroke patient may still be unstable and may be at risk for cerebral edema and brain herniation, complicating a clinical trial. While we lack extensive preclinical data on the optimal timing of cell therapy, one preclinical study suggests that IV mesenchymal stem cell (MSC) therapy may still have efficacy out to 1 month after stroke in rodents but there is a paucity of data at these later time points [18].
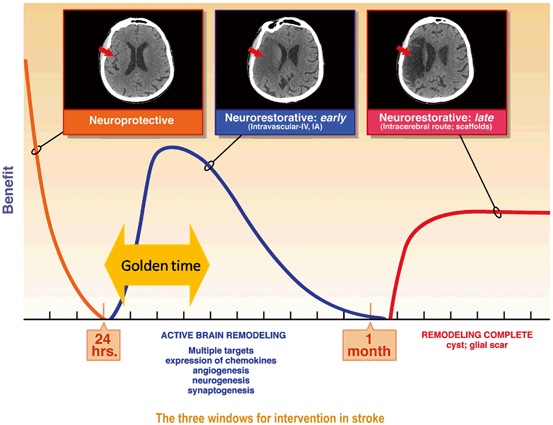

Fig. 1.2
The three main time windows for intervention: The acute neuroprotective window that closes rapidly in the first few hours, the “golden period” for reparative therapies where the brain is remodeling and there are immune system targets such as the spleen, and a later “chronic” period where the approaches will require intracerebral transplantation. From [15]
There are a wide variety of bone-marrow-derived stem cells (Fig. 1.3). To date, the most extensive preclinical support and clinical trial experience are with bone marrow mononuclear cells, MSC, and MultiStem, a proprietary plastic adherent cell. Autologous bone marrow mononuclear cells can be rapidly isolated and do not require expansion and can be delivered back into the patient within 72 h and clinical trials to date in acute stroke demonstrate safety [19]. This approach is adopted by Charles Cox and colleagues (Chap. 15) in their use of intravenous autologous bone marrow mononuclear cells in pediatric patients with moderate to serve traumatic brain injury. Taguchi and colleagues transplant autologous bone marrow mononuclear cells at a later “subacute” period of 7–10 days after stroke (see Chap. 4). They isolate bone marrow mononuclear cells from stroke patients between 7 and 10 days from stroke and reinfuse these autologous cells the same day. In a later time window of 1–3 months after stroke, Oh Bang (see Chap. 3) has completed a clinical trial, the STARTING trial, of intravenously transplanted autologous MSC, where autologous MSC were expanded ex vivo in bovine sera. The treatment was safe with hints of activity and subjects were followed up for 5 years. Oh Bang now reports the ongoing STARTING 2 trial where the MSC are expanded in the sera of autologous stroke patients to “condition” them prior to transplantation.
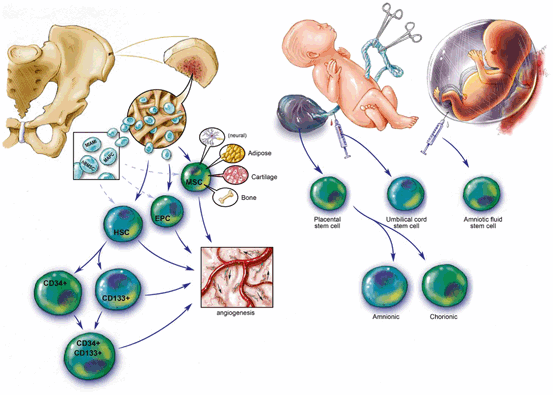

Fig. 1.3
Slide depicting the range of bone-marrow-derived cells and those derived from umbilical cord blood, placenta, and amniotic fluid
An alternative approach is to use an “off-the-shelf” allogeneic cell. This cell type requires neither human leukocyte antigen (HLA) matching nor any isolation from the stroke patient. These cells from a healthy donor are stored and “ready to go” allowing early administration in stroke patients. Athersys, Inc. has launched an early-phase clinical trial of MultiStem, an allogeneic “off-the-shelf” plastic adherent cell distinct from the MSC. This multicenter, multinational randomized double blind, placebo-controlled, double blind trial of 136 patients incorporated a dose escalation phase and will finish enrollment in late 2014/early 2015 [20]. This trial utilizes a cell dose of 1.2 billion cells per patient, a higher dose than other intravascular trials. This trial targets a “homogeneous” group of moderately severe stroke patients with cortical involvement and a baseline NIHSS from 8 to 20 in the 24–48 h period after stroke [20]. This early time window targets the end of the neuroprotective window and the early portion of the recovery window. The development of MultiStem is reviewed in Chap. 5 by Robert Mays.
With the increasing use and availability of IA interventional therapy with clot-retrieval devices and the “positive” results of the MR CLEAN clinical trial from the Netherlands, there will be more opportunity to deliver IA cell therapy, an approach used in many interventional cardiology trials. Yavagal and colleagues have pioneered using IA delivery of MSC with testing in a canine model and discuss the IA approach in Chap. 6
Preclinical studies suggest that intracerebral delivery of neural stem cells (NSC) and iPS cell-derived neural progenitor may also be more effective in the first week after stroke than at later time points. There remain logistical challenges for these approaches in this early time period, and the approach will not be as scalable as intravenous delivery approaches to community hospitals. Steinberg’s group at Stanford (Chap. 7) reviews the use of NSC and their own road to developing an NSC-based intracerebral therapy in human stroke. iPS-derived neural progenitor cells (iPS-NP) have great potential as a cell therapy and could be used in an autologous approach or more likely an allogeneic approach utilizing biobanks of HLA-matched cells. More work still needs to be done on the optimal timing and administration and there remains concern over the tumorigenic potential (see Chaps. 9 and 10). Unlike the intravascular approaches where significant cell engraftment does not occur, with these intracerebral approaches with NSC, there is cell engraftment with evidence that the transplanted cells integrate into brain circuitry as well as evidence that the cells provide beneficial paracrine effects on the host brain. It may also be possible to enhance the therapeutic effect of transplanted cells by using biodegradable matrices, discussed in Chap. 13 by Tom Carmichael’s group at UCLA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






