Levetiracetam
Michael D. Privitera
Jennifer Cavitt
Introduction
Levetiracetam, an antiepileptic drug (AED) with an interesting development history, has become widely used in the treatment of several types of epilepsy. Initial performance in standard animal screening models proved disappointing,34 but the drug’s success in clinical trials in humans have led some to question whether standard screening models should be reevaluated. Levetiracetam’s newly discovered mechanism of action is unique among AEDs, and has led to development of new compounds with binding at the SV2A site.45 For clinicians, levetiracetam has proved attractive because of its lack of hepatic metabolism, minimal drug interactions, activity against several seizure types, and ability to start an effective dose on day one.
Pharmacology
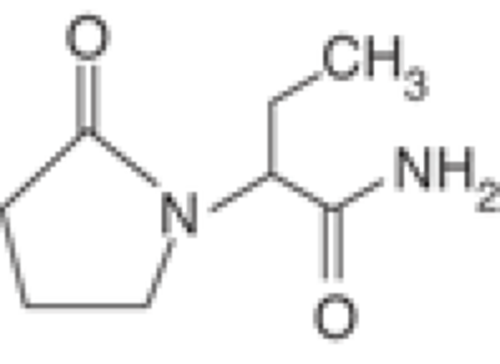
Levetiracetam—(S)-alpha-ethyl-2-oxo-pyrrolidine acetamide-—is a novel AED, chemically related to the nootropic agent, piracetam (Fig. 1).44
Standard animal models for screening and testing of AEDs indicate that levetiracetam may work differently than other AEDs. Klitgaard showed that levetiracetam did not stop seizures following acute maximal electrical shock (MES) and maximal pentylenetetrazol (PTZ) in rodents.34 However, these investigators showed that levetiracetam did protect against generalized seizures in rodents that had been kindled (i.e., animals who developed seizures due to chronic, submaximal stimulation with electrical shock or PTZ administration). Additionally, levetiracetam was ineffective in various maximal chemoconvulsive tests, except for protection against secondarily generalized activity from partial seizures induced by pilocarpine in mice and pilocarpine and kainic acid in rats. The antiseizure activity of levetiracetam persisted with chronic administration of methyl-6,7–dimthoxy-4–ethyl-beta-carboline-3–carboxylate, and it did not lower the seizure threshold of an inverse benzodiazepine receptor agonist. This pattern of activity differs greatly from that of other AEDs. An evaluation of levetiracetam’s major metabolite, (S)-alpha-ethyl-2–oxo-1–pyrrolidine acetic acid, using the same tests showed it to be inactive. There was a wide safety margin in normal and amygdala-kindled rats using the Rotorod impairment test.
Löscher et al. corroborated the findings of Klitgaard on the antiepileptogenic effects of levetiracetam in the kindling model of temporal lobe epilepsy.43 Only two of the older AEDs, valproate and phenobarbital, demonstrate mild antiepileptogenic properties in similar animal models for kindled seizures. The dose required to produce this effect is much greater, and the strength of the effect is much less for valproate and phenobarbital, compared with levetiracetam.
At the time of initial studies and U.S. Food and Drug Administration (FDA) approval, the mechanism of action of levetiracetam was unknown. Now, several lines of evidence indicate that levetiracetam exerts its antiepileptic action through binding at the synaptic vesicle SV2A receptor.45 Brain membranes and purified synaptic vesicles from mice lacking SV2A do not bind a tritiated levetiracetam derivative, indicating that SV2A is necessary for levetiracetam binding. Levetiracetam and related compounds bind to SV2A expressed in fibroblasts, indicating that SV2A is sufficient for levetiracetam binding. No binding was observed to the related isoforms SV2B and SV2C. A high degree of correlation occurs between binding affinities of a series of levetiracetam derivatives to SV2A in fibroblasts and to the levetiracetam-binding site in brain. Finally, a strong correlation exists between the affinity of a compound for SV2A and its ability to protect against seizures in an audiogenic mouse animal model of epilepsy. There appears to be some consensus that levetiracetam does not exert its antiepileptic activity through modulation or γ-aminobutyric acid (GABA) transmission or direct effect on sodium or calcium channels.57,63 These new and intriguing findings on levetiracetam’s mechanism have the potential to open a new avenue of investigation into proteins involved in vesicle exocytosis, and into SV2 in particular, as targets for the development of new AED therapies.
A study of levetiracetam’s efficacy in an experimental model of self-sustaining status epilepticus (SE) induced in rats by electrical stimulation revealed that intravenous pretreatment with levetiracetam reduced or prevented the development of self-sustaining seizures, and levetiracetam treatment during self-sustaining seizures decreased or aborted seizures.47 This study also found that levetiracetam significantly enhanced the anticonvulsant effects of diazepam, even at subtherapeutic doses.
Clinical Pharmacokinetics
Levetiracetam pharmacokinetics have been evaluated in pediatric, adult, and elderly patients, as well as in patients with renal and hepatic failure. Its rapid absorption, excellent bioavailability, minimal protein binding, lack of hepatic metabolism, and linear pharmacokinetics, make levetiracetam dosing straightforward.
Absorption
Following oral administration of 250 mg to 5,000 mg, the absolute bioavailability of levetiracetam is 95% to 100%.52 Peak plasma concentrations of approximately 31 μg/mL are achieved within 1 hour of a 1,000–mg oral dose. Repeated doses of 1,000 mg twice daily yield steady-state peak concentrations of approximately 43 μg/mL within 2 days. Peak concentrations and area under the plasma concentration-time curve (AUC) were linear for doses ranging from 500 to 5,000 mg in healthy volunteers. Food and antacids (calcium carbonate and aluminum hydroxide) slowed the rate of absorption, but did not decrease the extent of absorption. Additionally, there was no change in bioavailability with administration of a single 500–mg tablet compared with administration of two 250–mg capsules.
Table 1 Levetiracetam dosage adjustments in renal insufficiency (manufacturer’s recommendations) | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Plasma Protein Binding and Distribution
Levetiracetam appears to be distributed to intracellular and extracellular fluid with a volume of distribution of 0.5 to 0.7 L/kg.33 Less than 10% is bound to plasma proteins. Animal studies indicate that levetiracetam readily crosses the blood–brain barrier and appears to be evenly distributed throughout the brain.19
Metabolism and Elimination
Within 24 hours of an oral dose of levetiracetam, 93% of the drug has been excreted, 66% as unchanged drug in the urine and 27% as inactive metabolites.52 One major inactive metabolite, L057, accounts for 24% of the dose, and is formed in blood by hydrolysis of an acetamide group. This deamination process does not involve either the cytochrome P450 or UDP-glucuronyl transferase isozyme (UGT) systems. Two other minor metabolites account for 3% of the dose, and their metabolic pathway has not been determined.
The renal clearance of levetiracetam is 40 mL/min/1.73 m2 or 0.6 mL/min/kg and clearance of the major metabolite is 4.2 mL/min/kg.33 Levetiracetam apparently undergoes glomerular filtration with some tubular reabsorption, whereas L057 is actively secreted in renal tubules in addition to tubular reabsorption. Concomitant administration of probenecid increased concentrations of L057, but not levetiracetam. Because L057 is inactive, this change in elimination should not result in changes in response to levetiracetam. In normal adult volunteers, the elimination half-life of levetiracetam is 6 to 8 hours and is unchanged by doses up to 5,000 mg, route of administration, or frequency of administration. Steady-state concentrations are reached within 48 hours.52
Pediatric and Elderly Populations
In children with epilepsy who are 6 to 12 years old, the elimination half-life of levetiracetam is approximately 6 hours, and apparent total body clearance is 30% to 40% lower than adults.52 The peak concentrations and AUC adjusted for a dose of 1 mg/kg were also 30% to 40% higher than in adults. The fraction excreted unchanged in the urine was similar to that of adults, but the percent of L057 in the urine was lower in children. These values indicate that children may require doses that are 1.3 to 1.4 times a weight-normalized adult dose. For elderly patients, the elimination half-life is prolonged at 10 to 11 hours.31
Renal and Hepatic Impairment
The renal clearance of levetiracetam correlates directly with creatinine clearance, as does its primary metabolite.52 Patients with mild to moderate renal impairment (creatinine clearance of 20–89 mL/min/1.73 m2) had the total body clearance of levetiracetam decreased by 35% to 60%. Total body clearance was reduced by 68% in a patient with a creatinine clearance of <19 mL/min/1.73 m2. As expected, the steady-state peak concentration, half-life, and AUC increased with declining renal function. During hemodialysis, the elimination half-life for levetiracetam is approximately 25 hours for the interdialytic period and 3.1 hours for the intradialytic period. Approximately 50% of levetiracetam is removed during dialysis. Based upon these data, the manufacturer recommends dosage adjustments for varying degrees of renal function (Table 1).33
In subjects with mild (Child-Pugh A) to moderate (Child-Pugh B) hepatic impairment, the pharmacokinetics of levetiracetam were unchanged. In patients with severe hepatic impairment (Child-Pugh C), total body clearance was 50% that of normal subjects, but decreased renal clearance accounted for most of the decrease. No dose adjustment is needed for patients with hepatic impairment.33
Race and Gender
Levetiracetam Cmax and AUC were 20% higher in women (n = 11) compared with men (n = 12), however clearances adjusted for body weight were comparable. Formal pharmacokinetic studies of the effects of race have not been conducted. Cross-study comparisons involving whites (n = 12) and Asians (n = 12) show that the pharmacokinetics of levetiracetam were comparable between the two races. Because levetiracetam is primarily renally excreted and there are no important racial differences in creatinine clearance, pharmacokinetic differences due to race are not expected.31
Value of Plasma Concentrations
Plasma concentrations of levetiracetam were obtained in controlled trials. No study has demonstrated a relationship between plasma concentration and efficacy or adverse effects independent of dose. Levetiracetam does not undergo hepatic
metabolism, is not protein bound, and lacks drug interactions with most other medications; thus routine monitoring of plasma concentrations does not appear indicated.
metabolism, is not protein bound, and lacks drug interactions with most other medications; thus routine monitoring of plasma concentrations does not appear indicated.
Intravenous Formulation
The equivalence of levetiracetam injection and the oral formulation was demonstrated in a bioavailability study of 17 healthy volunteers.32 In this study, levetiracetam 1,500 mg was diluted in 100 mL 0.9% sterile saline solution and was infused over 15 minutes. The selected infusion rate provided plasma concentrations of levetiracetam at the end of the infusion period similar to those achieved at Tmax after an equivalent oral dose. It is demonstrated that levetiracetam 1,500 mg intravenous infusion is equivalent to three 500-mg oral tablets of levetiracetam. The time-independent pharmacokinetic profile of levetiracetam was demonstrated following a 1,500 mg intravenous infusion for 4 days with twice-daily dosing. The AUC(0–12) at steady-state was equivalent to AUCinf following an equivalent single dose. Equivalent doses of intravenous levetiracetam and oral levetiracetam result in equivalent Cmax, Cmin, and total systemic exposure to levetiracetam when the intravenous levetiracetam is administered as a 15-minute infusion.
Efficacy
Efficacy as Adjunctive Treatment in Partial Seizures
Levetiracetam is effective adjunctive treatment for medication-resistant partial seizures with or without secondary generalization. Several randomized, placebo-controlled trials have established that levetiracetam doses of 1,000 to 3,000 mg/day significantly reduce mean seizure frequency rates and produce significantly greater numbers of 50% responders when compared with placebo.
Shorvon et al.56 studied 324 patients, aged 16 to 65 years, with medication-resistant partial seizures and found that add-on levetiracetam significantly reduced seizure frequency compared with placebo, and 50% responder rates were significantly greater in levetiracetam groups compared with placebo. The median percentage reduction in seizure frequency was 17.7% and 26.5% for levetiracetam doses 1,000 mg/day and 2,000 mg/day, respectively, compared with 6.1% for placebo. In addition, a 50% reduction of 50% or greater in seizure frequency was seen in 22.8% of patients on levetiracetam 1,000 mg/day and 31.6% of patients on levetiracetam 2,000 mg/day, compared with 10.4% of patients taking placebo. However, no significant difference was found between levetiracetam doses of 1,000 mg/day and 2,000 mg/day. Betts et al.12 also failed to find greater efficacy with a higher dose of levetiracetam. In fact, in a study of 119 patients, aged 16 to 70 years, with medication-resistant epilepsy that included partial seizures with and without secondary generalization, as well as primary generalized tonic–clonic seizures, 2,000 mg/day add-on levetiracetam produced a significantly greater 50% responder rate (48.1%, p < 0.05) compared with placebo (16.1%). However, the 50% responder rate for patients on 4,000 mg/day levetiracetam (28.6%) was remarkably not significantly better than placebo, although no significant differences were found in seizure reduction by seizure type between the treatment groups.
In contrast, two other randomized, controlled trials did find a dose–response relationship for levetiracetam in medication-resistant partial seizures. In 324 patients, aged 16 to 65 years, studied by Boon et al.,13 both 1,000 mg/day and 2,000 mg/day doses of add-on levetiracetam significantly reduced mean seizure frequency and increased 50% and 75% responder rates compared with placebo. Specifically, 26.2% of patients on levetiracetam 1,000 mg/day and 34.3% of patients on levetiracetam 2,000 mg/day had a ≥50% reduction in seizure frequency, compared with 12.2% of those on placebo. Furthermore, the 50% responder rate with levetiracetam 2,000 mg/day was significantly greater than that with levetiracetam 1,000 mg/day (p = 0.018). Of patients receiving 1,000 or 2,000 mg/day levetiracetam, 13.7% and 20% of patients, respectively, had ≥75% reduction in seizure frequency, compared with 4% in the placebo group.
Similarly, Cereghino et al.15 found that both 1,000 mg/day and 3,000 mg/day add-on levetiracetam doses significantly reduced mean seizure frequency compared with placebo (32.5% and 37.1%, respectively, vs. 6.8% for placebo, p < 0.001) and significantly increased 50% responder rates compared with placebo in 294 patients aged 16 to 70 years. Of those taking 1,000 mg/day or 3,000 mg/day levetiracetam, 33% and 39.8%, respectively, had a ≥50% reduction in seizure frequency, compared with 10.8% of patients taking placebo. Moreover, a significantly greater number of patients in the 3,000 mg/day levetiracetam group (eight of 98 or 8.2%, p = 0.01), but not the 1,000 mg/day levetiracetam group (three of 94 or 3.2%) were seizure-free, compared with placebo (0 of 93).
Another randomized, controlled trial by Ben-Menachem et al.10 evaluated in 286 patients, aged 16 to 70 years, with medication-resistant partial seizures the efficacy of add-on levetiracetam 3,000 mg/day versus placebo. The investigators found that median seizure frequency, median percentage seizure reduction, and 50% responder rates were all significantly better in the levetiracetam group (1.06 seizures/week, 39.9%, and 42.1%, respectively) compared with the placebo group (1.75 seizures/week, 7.2%, 16.7%, respectively).
Ben-Menachem et al.9 also examined long-term efficacy of levetiracetam using data from 1,422 patients treated with levetiracetam in multiple studies. The investigators found that median percentage seizure frequency reduction did not decrease over time within cohorts of 6 to 54 months of levetiracetam exposure, suggesting that tolerance was not observed. In addition, 50% and 75% responder rates were similar (38.6% and 20.1%, respectively) for the whole treatment period of up to 54 months as those seen in randomized, controlled trials, indicating that short-term efficacy is sustained over a longer observation period as well.
French and Arrigo21 evaluated the rapid onset of action of levetiracetam in a pooled analysis of data from three randomized, double-blind, placebo-controlled trials of 883 patients, aged 16 to 70 years, with refractory partial seizures started on levetiracetam 1,000 or 333 mg/day versus placebo. The proportions of seizure-free patients in each group were analyzed for 3 days before and after starting levetiracetam or placebo. In the 1,000 mg/day levetiracetam group, the increase in the proportion of seizure-free patients over the day prior to treatment initiation was 15% for the first day of treatment, 17% for the second day of treatment, and 17% for the third day of treatment. All these differences were found to be statistically significant (p < 0.001). In the 333 mg/day levetiracetam group, the increase in the proportion of seizure-free patients over the day prior to treatment initiation was 7% for the first day of treatment, 9% for the second day of treatment, and 9% for the third day of treatment, but none of these differences was statistically significant. No major changes were observed in the proportion of seizure-free patients following initiation of placebo, with increases of 1%, 2%, and 1%, respectively, for days one, two, and three following treatment initiation, and these changes were also not statistically significant. Thus, rapid onset of action was noted when starting levetiracetam 1,000 mg/day.
A follow-up study22 examined the proportion of seizure-free days each week for the 3-month period following treatment
initiation for those same 883 patients to determine whether a change in efficacy occurs during the duration of the trial. A significantly greater mean proportion of seizure-free days was observed beginning with the first week after treatment initiation in the levetiracetam group compared with placebo. Moreover, a significantly greater mean proportion of seizure-free days was also seen for each week of the 3-month period, suggesting sustained efficacy of levetiracetam. Interestingly, the difference in mean proportion of seizure-free days between the levetiracetam and placebo groups was most pronounced in the first week after treatment initiation (0.81 for levetiracetam vs. 0.69 for placebo). However, the efficacy was stable for the following weeks in the 3-month period.
initiation for those same 883 patients to determine whether a change in efficacy occurs during the duration of the trial. A significantly greater mean proportion of seizure-free days was observed beginning with the first week after treatment initiation in the levetiracetam group compared with placebo. Moreover, a significantly greater mean proportion of seizure-free days was also seen for each week of the 3-month period, suggesting sustained efficacy of levetiracetam. Interestingly, the difference in mean proportion of seizure-free days between the levetiracetam and placebo groups was most pronounced in the first week after treatment initiation (0.81 for levetiracetam vs. 0.69 for placebo). However, the efficacy was stable for the following weeks in the 3-month period.
Krakow et al.35 analyzed the long-term continuation rate and efficacy of levetiracetam in 1,422 patients with medication-resistant epilepsy treated with adjunctive levetiracetam in several double-blind, placebo-controlled trials and open-label studies during the developmental program of the drug in Europe and the United States. Patients ranged in age from 5 to 78 years, but the majority of patients (95.2%) were 16 to 65 years old. The vast majority (93%) had localization-related epilepsy. Of 1,422 patients exposed to levetiracetam, 562 were still treated at the cut-off date. Kaplan-Meier survival analysis estimated continuation rates of 60%, 37%, and 32%, respectively at 1, 3, and 5 years. Adverse events led to treatment withdrawal in 225 of 1,422 patients (15.8%), and lack of efficacy led to withdrawal in 261 patients (18.4%). In 374 of 1,422 patients (26.3%), levetiracetam was discontinued due to reasons inherent in clinical trials, such as protocol violations, withdrawal of consent, study completed without starting open follow-up, lost to follow-up, and the like. Cox regression revealed four factors that significantly affected levetiracetam continuation: high maximum dose, low starting dose, presence of generalized seizures, and smaller number of AEDs at baseline. In 1,325 patients for whom seizure frequency data were available for both baseline and treatment periods, 512 (39.6%) had a ≥50% reduction in seizure frequency, 266 (20%) had a ≥75% reduction in seizure frequency, 183 (13%) were seizure-free for at least 6 months, 109 (8%) were seizure-free for at least 1 year, and 65 (4.5%) were seizure free from the first day of exposure.
Efficacy as Monotherapy in Partial Seizures
A large prospective trial demonstrated the effectiveness of levetiracetam as monotherapy. Ben-Menachem et al.8 studied the efficacy of 1,000 to 3,000 mg/day levetiracetam monotherapy versus 400 to 1,200 mg/day controlled-release carbamazepine monotherapy in 472 patients with newly diagnosed, localization-related epilepsy followed for at least 12 months in a randomized, double-blind, head-to-head trial. Six-month seizure freedom rates were similar in the levetiracetam (73.0%) and carbamazepine (72.8%) groups. Twelve-month seizure-freedom rates were 56.6% in the levetiracetam group and 58.5% in the carbamazepine group. Significantly fewer patients taking levetiracetam (16.1%) discontinued therapy or had a dose change due to an adverse event compared with carbamazepine (23.0%).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree