Levetiracetam
Joseph I. Sirven
Joseph F. Drazkowski
Levetiracetam (LEV) is a novel antiepileptic drug (AED) approved in 2000 by the US Food and Drug Administration as adjunctive therapy for patients with partial epilepsy. The compound was developed as a derivative of the nosotropic agent piracetam, with a wide spectrum of anticonvulsant effects in animal models of various types of epileptic seizures (1). It is chemically unrelated to existing AEDs. In addition to its unique chemical structure, LEV has a distinct mechanism of action and a favorable pharmacokinetic and safety profile, making it an attractive therapy for seizure management.
CHEMISTRY
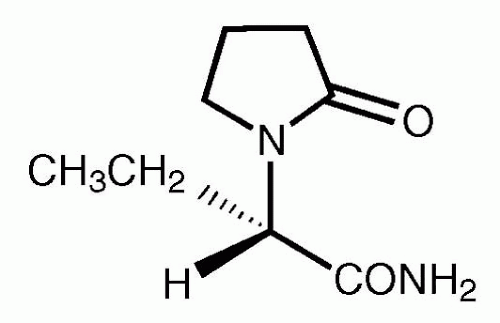
LEV is a single enantiomer (-)-(S)-α-ethyl-2-oxo-1-pyrrolidine acetamide with a molecular weight of 170.21 (1,2). The structural formula of the agent is shown in Figure 63.1. The drug is a white to off-white crystalline powder with a faint odor and bitter taste. It is very soluble in water (104.0 g/100 mL), freely soluble in chloroform and in methanol, and soluble in ethanol. It is much less soluble to insoluble in acetonitrile and n-hexane. Keppra* (the only brand name of this compound) tablets contain LEV and the inactive ingredients silicon dioxide, cornstarch, methylcellulose, magnesium stearate, polyethylene glycol 4000, and coloring agents. LEV is supplied as 250-mg (blue), 500-mg (yellow), and 750-mg (orange) tablets (2). Currently, an oral suspension is undergoing clinical investigation for approval in the United States.
MECHANISM OF ACTION
Prior to undergoing standardized AED testing by the National Institutes of Health, LEV was found to have antiepileptic properties. In contrast to all approved AEDs, LEV lacked conventional modulation of the acute seizure model (maximum electroshock seizure [MES] test and pentylenetetrazol [PTZ]), suggesting a novel mechanism of action (3, 4, 5). Moreover, LEV displays unique potent protection against kindled seizures in both mice and rats during kindling models (3,4). In comparative tests with established AEDs in a number of animal models of epileptic seizures, LEV displays potent protection in a broad range of animal models of chronic epilepsy, including partial and primary generalized seizures (5).
The precise mechanism by which LEV exerts its AED effect is unknown. It does not appear to derive its function from known mechanisms involved in inhibitory and excitatory neurotransmission, but it may be active at a brain-specific binding site (6). A stereoselective binding site for LEV has been shown to exist exclusively in membranes from cells in the central nervous system (CNS), but not in peripheral tissue (3,6). There is no significant displacement (≤10 mM) of ligands specific for 55 different binding sites. Established AEDs, such as carbamazepine, phenytoin, valproate, phenobarbital, and clonazepam, do not possess an affinity for this binding site (6).
In studies performed to demonstrate the cellular pharmacodynamics of LEV, the agent reduces calcium current through neuron-specific, high-voltage-activated N-type calcium channels, thus reducing seizure potential (7). It does not modulate neuronal voltage-gated sodium, T-type calcium currents, or glutamate receptor-mediated neurotransmission in the spinal cord, nor does it have any conventional effects at the gamma-aminobutyric acid (GABA)a receptors (7). However, LEV does promote inhibitory neurotransmission by reducing negative allosteric effects of zinc and the beta-carbolines on GABAa and glycine receptors (8). In vitro and in vivo recordings of epileptiform activity from the hippocampus have shown that LEV inhibits burst firing without affecting normal neuronal excitability, suggesting selective suppression of hypersynchronization
of epileptiform burst firing and propagation of seizure activity (9). The only certainty regarding the mechanism of action is that further investigation is warranted to elucidate the ways in which LEV exerts its selective effects.
of epileptiform burst firing and propagation of seizure activity (9). The only certainty regarding the mechanism of action is that further investigation is warranted to elucidate the ways in which LEV exerts its selective effects.
ABSORPTION, DISTRIBUTION, AND METABOLISM
Overview
LEV is rapidly and almost completely absorbed following oral administration. The pharmacokinetics are linear and time invariant, with low individual variability (10). LEV is not protein-bound (less than 10%), and its volume of distribution is close to the volume of intracellular and extracellular water (10,11). Sixty-six percent of the dose is unchanged as it is excreted renally (10). The major metabolic profile of LEV is an enzymatic hydrolysis of the acetamide group (10,11). LEV is not liver cytochrome P450-dependent (10). Its metabolites have no known pharmacologic activity and are renally excreted. The plasma half-life of LEV across studies is approximately 6 to 8 hours. The effects of the agent are increased in the elderly (primarily due to impaired renal clearance) and in patients with renal impairment (10,11).
Absorption and Distribution
Absorption of LEV is rapid, with peak plasma concentrations occurring about 1 hour following oral administration. Oral bioavailability is 100%, with no effect from ingestion of food. Linear pharmacokinetics characterize LEV over a dose range of 500 to 5000 mg. Steady state is achieved after 2 days of multiple twice-daily dosing. LEV is less than 10% bound to plasma proteins; clinically significant interactions with other drugs through competition for protein-binding sites are unlikely (10,11).
Metabolism and Elimination
LEV is not extensively metabolized in humans with the major metabolic pathway of enzymatic hydrolysis of the acetamide group, which produces the pharmacologically inactive carboxylic acid metabolite. There is no dependency on P450 cytochrome liver metabolism (10,11).
LEV is eliminated by renal excretion as unchanged drug, which represents 66% of the administered dose (10). The total body clearance is 0.96 mL/min/kg and the renal clearance is 0.6 mL/min/kg (10,11). The mechanism of excretion is glomerular filtration with subsequent partial tubular reabsorption. Elimination is correlated with creatinine clearance (10).
Special Populations
Pediatrics
The pharmacokinetics of LEV have been evaluated in children 6 to 12 years of age following single 20-mg/kg doses. The apparent clearance of LEV was approximately 40% higher in children than in adults. The half-life in children is 4 to 8 hours, compared with approximately 7 hours in adults. The maximum concentration of drug (Cmax) and area under the curve (AUC) values are comparable to those in adults. There is no correlation between age and gender among pediatric patients (11).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree