Chapter 149 Light Therapy
Abstract
Exposure of the eyes to light of appropriate intensity and duration, at an appropriate time of day, can have marked effects on the timing and duration of sleep and on the affective and physical symptoms of depressive illness.1 Here we review and evaluate delivery systems and the application of light therapy for circadian rhythm sleep disorders including delayed sleep phase disorder (DSPD), advanced sleep phase disorder (ASPD), and free running (or non–24-hour) sleep disorder. The most extensive clinical trials have focused on wintertime recurrence of major depression (seasonal affective disorder) [SAD]. We also cover light therapy for nonseasonal depressions (recurrent, chronic, bipolar), including combination treatment with sleep deprivation (wake therapy) and antidepressant medication. Light therapy holds promise in treating several clinical disorders.1a–1f
Light Delivery
Apparatus
Light Boxes
Factors include lamp type (output intensity and spectrum), filter, ballast frequency (for fluorescent lamps), size and positioning of radiating surface, heat emission, and so on. One clinically tested model (Fig. 149-1) illustrates modifications in second-generation apparatus, including smaller size, portability, raised and downward-tilted placement of the radiating surface to reduce glare, height adjustment relative to the patient’s head, a smooth polycarbonate diffusion screen with maximal ultraviolet (UV) filtering, and high-output fluorescent lamps (nonglaring 4000-Kelvin color temperature) driven by high-frequency solid-state ballasts. The combination of elements in this configuration yields a maximum illuminance of approximately 10,000 lux with the patient seated in a position with the eyes about 30 cm (1 foot) from the screen.

Figure 149-1 Table-mounted, tilted, height-adjustable, 10,000-lux, UV-filtered, diffused 4000-Kelvin fluorescent light system.
(Photograph courtesy of the Center for Environmental Therapeutics, http://www.cet.org.)
With the direction of gaze downward toward the work surface, such a configuration provides pleasant illumination suitable for reading and, despite illuminance far higher than in normal home lighting, is generally well tolerated (see Adverse Effects of Bright Light Exposure, later). The presentation of light from above eye level is supported by a study showing enhancement of melatonin suppression with directional illumination of the lower retina.2 As the apparatus becomes miniaturized, however, the field of illumination narrows, and even small changes in head position can substantially reduce the intensity of light that reaches the eyes.
Claims for the specific efficacy of any particular lamp type or spectral distribution, although commonly made, need to be questioned. Unfortunately, systems have been marketed that provide excessive visual glare, exposure of naked bulbs, direct intense illumination from below the eyes (“ski slope” effect), and intentionally augmented short-wavelength blue and UV radiation. The earliest light therapy trials used full-spectrum white fluorescent light at high color temperature with increased blue and near-UV radiation, in an attempt to approximate the spectral distribution of skylight relative to standard fluorescent sources. UV, however, was shown to be unnecessary for the antidepressant effect,3 leading to the use of alternative light sources and light boxes with UV filters to reduce exposure hazard to the eyes and skin. Claims that blue light is selectively therapeutically active4 have not yet been substantiated. Full-spectrum lighting is tolerable at 2500 lux, but aversive glare is excessive at 10,000 lux, the dose designed to reduce average session duration from 2 hours to 30 minutes.5 Softer white light with lower color temperature (3000 to 4000 Kelvin) works as well. Both the clinician and consumer must be vigilant in the selection of apparatus. Criteria are reviewed on the therapy Web page of the nonprofit Center for Environmental Therapeutics, http://www.cet.org.
Dawn Simulators
Dawn simulation methodology provides a major contrast to bright light therapy. A microprocessor-controlled lighting device delivers a mimic of gradual twilight transitions found outdoors in the spring or summer. A laboratory study of healthy young adults demonstrated that the addition of simulated, naturalistic dawn exposures blocked the delay drift of circadian rhythms under dim light–dark cycles, when subjects were awakened to view the signal.6 For clinical use, relatively dim dawn signals are presented to the patient while asleep, when the eyes are adapted to the dark and the circadian system is most susceptible to phase advances (see Timing of Morning Light Exposure, later). The initial open-label studies of dawn simulation found an antidepressant response; normalization of hypersomnic, phase-shifted, and fractionated sleep patterns; phase advances of the melatonin cycle; and truncation of melatonin production after simulated sunrise.7–9
Avery and colleagues10 conducted a 6-week controlled clinical trial of sigmoid light-onset ramps (which differ from the curvilinear acceleration of naturalistic dawns) rising to 250 lux between 4:30 AM and 6:00 AM, which were significantly more antidepressant than dim red control signals rising to 0.5 lux. The treatment was superior to postawakening light therapy at 10,000 lux administered between 6:00 AM and 6:30 AM (which may have been too early for these phase-delayed patients). Terman’s group11 conducted a controlled trial of naturalistic dawn simulation ending at individually selected wake up times and found similar rates of improvement to postawakening bright light therapy, and both methods were superior to low-density negative air ions, a nonphotic placebo. The average posttreatment phase advance of dim light melatonin onset was 0.58 hours, in contrast to a small delay shift of 0.19 hours under placebo (2-tailed t-test, P = .001).11a
Safety of Bright Light for the Eyes
Ophthalmologic evaluations of unmedicated patients with normal oculoretinal status have thus far shown no obvious acute light-induced pathology or long-term sequelae.12 Although the intensity of bright light treatment falls well within the low outdoor daylight range, the exposure conditions differ from those outdoors, and prolonged use entails far greater cumulative light exposure than is normally experienced by urban dwellers and workers.13,14
There remains a risk of direct retinal photoreceptor and pigment epithelium damage, drug photosensitization, and acceleration of age-related macular degeneration to visible spectral components above the UV range up to about 500 nm (blue light).15–17 Although wavelengths from ∼450 to 500 nm preferentially suppress nocturnal melatonin production18 and enhance circadian phase shifting,19 the selective therapeutic benefit of such light has yet to be ascertained. Nevertheless, manufacturers have marketed a variety of blue-light devices. Because the blue-light hazard is magnified at wavelengths below 450 nm, ideally these should be completely filtered out, although commercial fluorescent apparatus has yet to do so. Clinical studies of phase shifting20 and antidepressant effect21 with blue light thus far have shown no advantage over broad-spectrum white light, which remains the standard. White light, of course, includes a blue component, though in lower relative proportion than from narrow-band or blue-supplemented sources. The potential adverse effects of concentrated short-wavelength radiation suggest that clinical implementation for long-term treatment is problematic.22
At the opposite end of the light spectrum, ocular exposure to infrared illumination, which makes up about 90% of the output of incandescent lamps, poses risk of damage to the lens, cornea, retina, and pigment epithelium.23 Thus, despite being marketed for light therapy, incandescent lamps are contraindicated.
Light-box diffusion filters vary widely in short-wavelength transmission (for examples, see reference 24). Transmission curves should be demanded of manufacturers and compared with published standards. Normal clouding of the lens and ocular media that begins in middle age, as well as formation of cataracts, serves to exacerbate perceptual glare that can make high-intensity light exposure quite uncomfortable.24
Furthermore, both UV and short-wavelength blue light can interact with photosensitizing medications—including many standard antidepressant, antipsychotic, and antiarrhythmic agents, as well as common medications such as tetracycline—to promote or accelerate retinal pathology, whether acute, or slow and cumulative.23 In one reported case, a patient received combination treatment with clomipramine and full-spectrum fluorescent light. After 5 days, the patient had reduced contrast sensitivity, foveal sensitivity, and visual acuity and central scotomas and lesions, fortunately with only minor residual aftereffects in contrast sensitivity and scotoma 1 year after discontinuation.25
Filtered glasses are available (see Resources, later) that eliminate transmission of short-wavelength blue light while maximizing exposure above the range of primary circadian photoreception (>535 nm), reducing glare, enhancing visual acuity and subjective brightness,26 and minimizing the risk of drug photosensitization. Such protection is clinically useful to forestall circadian rhythm phase delays and melatonin suppression27 when patients are exposed to ambient light, especially before sleep, with increased risk of initial insomnia.
Although there are no definite contraindications for bright light treatment other than the retinopathies, research studies have routinely excluded patients with glaucoma or cataract. Some of these patients have used light therapy effectively in open treatment; this should be done, however, only with ophthalmologic monitoring. A simple eye checkup is advised for all new patients, for which a structured examination chart has been designed (see Resources, later).28 The examination has occasionally revealed preexisting ocular conditions that should be distinguished from potential consequences of bright light treatment.
Adverse Effects of Bright-Light Exposure
The earliest clinical trials of 2500-lux full-spectrum fluorescent light therapy for winter depression noted infrequent side effects of hypomania, irritability, headache, and nausea.29,30 Such symptoms often subside after several days of treatment. If persistent, they can be reduced or eliminated by decreasing the dose. Rarely have patients discontinued treatment due to side effects.
Two cases of induced manic episodes have been reported in drug-refractory nonseasonal unipolar depressives beginning after 4 to 5 days of light treatment.31 A few cases of light-induced agitation and hypomania have been noted, also in patients with nonseasonal depression.32 A patient with seasonally recurrent brief depressions developed rapid mood swings after light overexposure (far exceeding 30 minutes per day at 10,000 lux),33 and a patient who had unipolar winter depression and similar exposure showed his first manic episode34; both patients required discontinuation and medication. We had one bipolar patient with winter depression who became manic after the use of light and was given lithium as an effective countermeasure; almost all patients using mood stabilizers have responded to light therapy without mania. However, some have switched into mixed states with early morning light exposure, which was resolved by moving treatment to midday.35 Three cases of suicide attempt or ideation, also occurring in patients with winter depression, were reported within 1 week of standard early-evening bright-light treatment, and the patients required hospitalization.36
A 42-item side-effect inventory was administered to 30 patients with winter depression after treatment with unfiltered full-spectrum fluorescent light at 2500 lux for 2 hours daily.37 Other than for one case of hypomania, there were no clinically significant side effects. Patients given evening light (the timing relative to bedtime was unspecified) reported initial insomnia. Mild visual complaints included blurred vision, eyestrain, and photophobia.
Of specific interest is the side-effect profile for patients using a downward-tilted fluorescent light box protected by a smooth diffusion screen (see Fig. 149-1), with 30-minute daily exposures at 10,000 lux, because this method has had widespread application. A study of 83 patients with winter depression who were evaluated for 88 potential side effects38 identified a small number of emergent symptoms at a frequency of 6% to 16%, including nausea, headache, jumpiness or jitteriness, and eye irritation.39 These results must be weighed against the improvement of other patients who showed similar symptoms at baseline but became asymptomatic after light treatment: All symptoms, except nausea, showed greater improvement than exacerbation, which forces attention to the risk-to-benefit ratio. Indeed, emergence of symptoms might have reflected the natural course of the depression in nonresponders to light, rather than a specific response to light exposure.
Case Management, Timing, and Dosing
Monitoring of Patients
Light treatment is typically self-administered at home on a schedule recommended by the clinician. To the extent that the timing of light exposure is important to obtain a therapeutic effect, patient adherence is a sine qua non. The patient and clinician need to work together to find the optimum dosing–timing combination. At the Columbia clinic, the two talk by telephone after the first 3 days of treatment, although the patient is expected to report any side effects the same day they are experienced. Patients maintain a daily sleep, mood, and energy log (downloadable in hard copy or PC formats),40 which they are asked to email or fax before subsequent scheduled contacts. This information is essential for tracking sleep-phase changes in response to the treatment, and adjusting the dose or timing. Patients with depression also complete a hard copy or online symptom rating scale (see Resources, later). With the personalized feedback presented online, this exercise also teaches patients to track their status independently, in preparation for self-management of light therapy after the initial period of guided treatment.
Timing of Morning Light Exposure
The thrust of recent clinical trials (see Seasonal Affective Disorder, later) leads to the recommendation that patients with winter depression initially be given morning light shortly after awakening. The dose of 10,000 lux for 30 minutes5,41 appears to be most efficient. A similar strategy applies to patients with DSPD, with evening light for ASPD, but longer exposure duration up to 60 minutes may been needed. Although intensities as low as 2500 lux can also be effective, they require longer exposure,42,43 and to accommodate such treatment in the morning most patients would have to wake far earlier than their habitual wake up time, with a risk of counterproductive circadian phase delays.
The advantage of morning light appears to lie in circadian rhythm phase advances, which can be measured as shifts in the time of nocturnal melatonin onset.44 The magnitude of the antidepressant response varies with the magnitude of phase advances. In a protocol with 10,000-lux treatment for 30 minutes on habitual awakening, the magnitude of antidepressant response was negatively correlated with the interval between dim-light melatonin onset (DLMO) and treatment time (r = −0.38, P = .01).45 The greatest improvement was seen with light therapy 7.5 hours after DLMO, which produced a 2.7-hour phase advance over 3 weeks. Overall, light therapy given 7.5 to 9.5 hours after melatonin onset yielded twice the remission rate (80% versus 38%) of light given 9.5 to 11.0 hours after DLMO.46 Clock time per se should not be used to schedule morning light administration, because the baseline DLMO—our metric for circadian time—spans a 7-hour range.
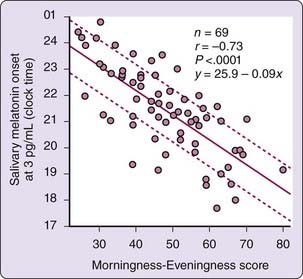
Unfortunately, a phase diagnostic based on melatonin assays is not readily available in clinical practice. The melatonin assay remains primarily a research tool, with high cost and slow turnaround time, although user-friendly clinical kits are becoming available. To provide the clinician a basis to readily specify treatment time, a quick, approximate solution lies in the relation between melatonin onset and the Horne-Östberg Morningness–Eveningness Questionnaire (MEQ)47 score, which for unmedicated winter depression patients are strongly correlated (r = −0.73; Fig. 149-2). Healthy subjects without depression show a similar relationship.48
One thus can schedule morning light exposure at individually determined circadian times by estimating the time of melatonin onset from the MEQ score, a strategy that facilitates circadian rhythm phase advances and the antidepressant response. Given the spread of DLMOs around the regression line (see Fig. 149-2), there is a risk that light will be scheduled too early, which might lead to premature awakening or counterproductive phase delays. The MEQ-based recommendation maximizes the chance that the patient will receive light in the window of 7.5 to 9.5 hours after DLMO. With a target time of 8.5 hours after DLMO, most patients (44/69, or 64% in our sample) will be captured in this window (± 1 hour) and 66/69 (96%) will receive light within ± 2 hours.
A list of recommended initial light exposure times, derived from the regression of the MEQ score on melatonin onset for 8.5 hours after DLMO, is shown in Table 149-1 (An online version of the MEQ,49 which has been used successfully for more than 6 years, automatically returns the recommended light exposure interval to the user.) This schedule is a best-guess solution, and prompt timing adjustments may be needed. For example, if the circadian phase estimate were too early, a patient might report an uncontrollable urge to resume sleep for several hours after treatment (unintended phase delay), or sleep-onset insomnia beyond habitual bedtime, in which case light exposure should be moved later to bring the session into the 7.5 to 9.5 hour post-DLMO range. Similarly, if the patient suddenly starts waking up hours earlier than expected, the session should be moved later. On the other hand, with light exposure later than 9.5 hours after DLMO, there might be inadequate response. If 5 days of treatment shows no sign of improvement, the light should be moved earlier.
Table 149-1 Timing of Morning Light Therapy* Based on Morningness–Eveningness Score
| MEQ SCORE | START TIME |
|---|---|
| 16-18 | 08:45 |
| 19-22 | 08:30 |
| 23-26 | 08:15 |
| 27-30 | 08:00 |
| 31-34 | 07:45 |
| 35-38 | 07:30 |
| 39-41 | 07:15 |
| 42-45 | 07:00 |
| 46-49 | 06:45 |
| 50-53 | 06:30 |
| 54-57 | 06:15 |
| 58-61 | 06:00 |
| 62-65 | 05:45 |
| 66-68 | 05:30 |
| 69-72 | 05:15 |
| 73-76 | 05:00 |
| 77-80 | 04:45 |
| 81-84 | 04:30 |
| 85-86 | 04:15 |
MEQ, Horne-Östberg Morningness–Eveningness Questionnaire.
* Start of 10,000-lux, 30-minute session, approximately 8.5 hours after estimated melatonin onset. Bold indicates the range confirmed in clinical trials.
Data from Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr 2005;10:647-663.
The MEQ solution is not diagnostic of circadian phase.50 For example, as shown in Fig. 149-2, it is possible for individual evening types (score below 42) and morning types (score above 58) to share the same DLMO.51 Another source of error, of course, lies in the DLMO measure itself.48 The MEQ algorithm offers the clinician an intelligent starting point that varies substantially from patient to patient, minimizing the problem of inappropriate treatment times, which would otherwise often be too early or too late.
Although the algorithm is based on winter depression data, it has been applied successfully to patients with nonseasonal unipolar and bipolar depression52 and moderate delayed sleep phase. However, for DSPD patients going to sleep after 2 AM, night shift workers, or the retired elderly, several questions on the MEQ will be difficult or impossible to answer, and timing decisions should be based on a pretreatment sleep log instead. Additionally, the MEQ score may be distorted by masking effects of hypnotic medication, compromising its usefulness as a circadian phase estimator.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree