Fig. 26.1
A 45-year-old man with small bowel neuroendocrine tumor, with left hepatectomy for liver metastasis. (a) Axial CT scan (arterial phase) shows new metastatic liver lesion in the remnant right liver. (b) Axial CT scan (portal venous phase) obtained after radiofrequency ablation of the lesion shows hypoattenuation with no residual lesion
RFA can be performed percutaneously under CT or US guidance or intraoperatively mostly in combination with liver resection using either laparoscopic or open approach.
Classical indications of RFA are LM fewer than five lesions and tumor size less than 5 cm [5]. Yet, two other issues should be discussed in LM from NET: the role of RFA in tumor debulking and in controlling functional syndromes due to specific hormones excess. This explains that most series of patients with LM from NET had more than five ablated tumors with intraoperative RFA during one session [9, 10].
In Elias’s series, 16 patients had combined liver surgery and RFA [9]. A mean of 15 and 12 LM per patient were surgically removed and RF ablated, respectively. Morbidity was observed in 69 % of the cases. The 3-year overall survival and disease-free survival were similar to their previous experience of liver resection alone of LM from NET.
In Akyildiz’s series, 119 laparoscopic RFA without liver resection were performed in 89 patients with LM from NET. The mean tumor size was 3.6 cm, and the mean number of tumors was 6 (range 1–16) [10]. Perioperative morbidity was 6 %, and 30-day morbidity was 1 %. Forty-four patients had hormonal symptoms prior the procedure. One week after RFA, 97 % of these patients reported at least partial symptoms relief, and 73 % had significant or complete relief. The symptomatic response lasted for a median of 14 ± 5 months [10]. Median disease-free survival was 1.3 year, and overall survival was 6 years after RFA.
Follow-up by imaging (CT and or MR imaging) is essential to assess complete tumor necrosis. One of the major problems is the recurrence of metastases within the liver as new tumors are reported up to 63 % in the largest series of patients treated with RFA [10]. Conversely, local liver recurrence was observed from 3.3 to 7.9 % per lesion [10, 11]. Interestingly, in a meta-analysis including 5.224 ablated tumors of various origin, the rate of local recurrence was lower in neuroendocrine LM than in others [11]. This might be due to tumor characteristics such as well-circumscribed margins or to natural history of these tumors [11]. As in other liver malignancies, factors predictive of tumor recurrence are tumor size, ablation margin, and blood vessel proximity [12]. In a multivariate analysis, statistically significant determinants of survival were only gender (with males having the worse prognosis) and size of the dominant liver metastasis (a tumor size exceeding 3 cm was associated with a greater mortality) [13].
Complications observed after RFA are not related to tumor type. They include pain, bile leakage, liver abscess, intra-abdominal hemorrhage, bowel perforation, and pulmonary complications [5, 10, 12, 13].
In patients with LM who had previous Whipple procedure and bilioenteric anastomosis, we have to keep in mind that RFA dramatically increases the risk of liver abscess formation (40 % vs. 0.4 %) [12].
In summary, RFA of LM from NETs differs from other liver metastases due to the large number of lesions per patient. Then RFA is mostly palliative aiming at debulking and controlling hormonal symptoms. This explains why intraoperative approach with or without combined liver resection is preferred rather than percutaneous approach.
26.2.2 Microwave Ablation
MWA uses electromagnetic devices with frequencies ≥900 MHz. The principle of this technique is similar to RFA but has several theoretical advantages. First, the intratumoral temperatures are consistently higher than can be achieved with RFA. Second, MWA is overcoming the “heat-sink” effect observed in RFA due to the cooling effect of blood flow in large vessels close to the tumor, both resulting in a better tumor control.
MWA has not been extensively evaluated in LM from NET. Only one series reported 11 patients with LM from NET out of 100 patients [14]. As with RFA, most procedures were performed intraoperatively either with concomitant hepatic resection (7/11) or concomitant extrahepatic tumor resection (6/11). The median number of ablated LM was 4 ranging from 1 to 13 tumors. Complications were observed in three patients. No local liver recurrence was noticed [14].
26.2.3 Cryotherapy
Cryotherapy is based on the decreased cell viability at low temperatures. The obtained tissue temperature should be −50 °C to achieve necrosis in neoplastic tissue.
To our knowledge, only three series have evaluated cryotherapy in LM from NETs (the largest with 19 patients) [15–17]. As with other thermal ablative techniques, hormonal symptom relief was observed in the vast majority of patients. Notably, postprocedural coagulopathy has been found in all patients of the two main series requiring transfusion of either platelets or fresh frozen plasma [15, 16]. In one of these series, two patients required intra-abdominal packing and transfusion of clotting factors [16]. The authors have not observed similar complications in any other liver malignancies and speculated that the necrosing carcinoid tumors were releasing substances that may disrupt the coagulation cascade [16].
Despite the efficacy on hormonal symptoms, cryotherapy has been gradually replaced by RFA, mainly for safety reasons.
26.3 Transarterial Chemoembolization and Bland Embolization
26.3.1 Rationale and Results
The rationale for transarterial hepatic embolization (TAE) is based on the fact that most LMs from NET are hypervascular and derive their blood supply from hepatic artery. The goal of TAE is to induce ischemia of tumor cells thereby reducing hormone output and causing necrosis. Various particles have been used including gelfoam, polyvinyl alcohol particles, and more recently, microspheres.
In the 1990s, transarterial chemoembolization (TACE) has been developed based on the principle that ischemia of the tumor cells increases sensitivity to chemotherapeutic substances [18]. Another advantage of TACE over TAE is the higher drug concentration obtained by regional delivery of chemotherapy. In TACE, embolization is performed immediately after intra-arterial injection of cytotoxic agents.
Despite the large number of TACE or TAE studies performed in patients with LM from NET, there are no randomized trials. Most of studies have evaluated clinical, biological, and morphological responses. Partial or complete symptom relief was observed in 42–100 % which lasts between 9 and 24 months [19, 20]. Significant decrease in tumor markers occurred in 13–100 % [19, 21]. Morphological response (either complete or partial) was seen in 8–94 % [21, 22]. Yet, imaging criteria for assessing tumor response have not been detailed in all published articles. When evaluated, overall survival since TAE or TACE initiation ranges from 15 to 80 months [23, 24].
Predictive factors of tumor response after TAE or TACE have been identified. Some of them depend on LM characteristics such as tumor liver involvement <30 % and tumor enhancement on arterial phase CT images [25, 26]. Primary tumor of the jejunum/ileum is associated with a better tumor response of the LM than pancreatic tumor [23, 25–28].
26.3.2 Technical Issues
Careful analysis of the literature highlights many disagreements on technical issues.
Such as choice between TAE and TACE is not clear. Several studies have retrospectively compared TAE and TACE in patients with LM from NETs. In all studies but one, treated patients had NET from the jejunum/ileum and NET from pancreatic origin, and no subgroup analysis has been performed. In two studies, no differences have been shown in terms of patient survival and tumor response [29, 30]. In one study, chemoembolization demonstrated trends toward improvement, in time to progression, symptom control, and survival (although not significant) [31]. Furthermore, these authors, as others, have shown that chemoembolization was not associated with a higher degree of toxicity than bland embolization [31, 32]. Gupta et al. have separately analyzed their results in small intestinal tumors and pancreatic tumors. They have shown that the addition of intra-arterial chemotherapy to embolization did not improve the overall survival nor progression-free survival in patients with small intestinal tumors. Moreover, it had a deleterious effect on the morphologic response rate [27]. In contrast, a tendency toward prolonged survival and improved response rate was noted in patients with pancreatic tumors treated with TACE compared to TAE [27]. A prospective comparison between TAE and TACE in neuroendocrine LM from the midgut has been published recently [29]. Primary endpoint was progression-free survival. The expected number of enrolled patients was not achieved explaining that this study may suffer from a lack of power. Yet, no difference was seen in the two groups [29]. The 1st year progression-free survival rates were 91.6 and 90 % in the TAE and TACE arms, respectively. The median PFS was 24 and 19 months in the TAE and TACE arms, respectively. There results confirm that the addition of intra-arterial chemotherapy to embolization does not prolong PFS. In summary, TACE has not been proved superior to TAE in LM from the jejunum/ileum. The question is still open in LM from neuroendocrine tumors of the pancreas.
It is known that embolization stimulates release of VEGF into the circulation. Authors have speculated that sunitinib, an oral VEGFR inhibitor, could be administered following embolization [33]. They observed high rates of PFS (15.2 months) and OS (95 and 59 % at 1 and 4 years, respectively) associated with this sequence of therapies.
Most cytotoxic drugs that have been injected during TACE procedure are drugs that are currently used with systemic chemotherapy. Various drugs have been used: doxorubicin and streptozotocin being the most common injected and, alone or in combination, mitomycin C, cisplatin, and gemcitabine. Even some teams have injected a mixture of doxorubicin, mitomycin, and cisplatin. Most teams recommend doxorubicin in small intestinal tumors and streptozotocin in pancreatic tumors [24, 25]. As drug assignment was not controlled nor randomized, it is not possible to determine which drug is more efficient. However, authors see potential advantage in using streptozotocin, especially in LM from the pancreas, which may save doxorubicin for subsequent use and chemotherapy [25] (Fig. 26.2). As injection of streptozotocin has been reported to be painful, the procedure is then performed under general anesthesia [34].
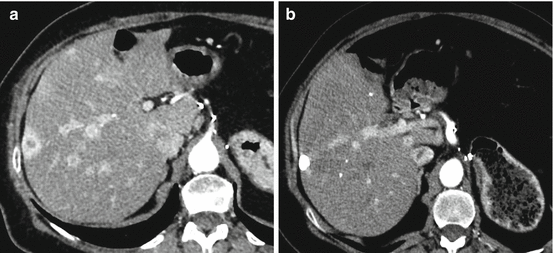

Fig. 26.2
A 76-year-old man with pancreatic neuroendocrine tumor and multiple liver metastases. (a) Axial CT scan shows hypervascular lesions at the arterial phase. (b) Axial CT scan after chemoembolization using streptozotocin shows a major lipiodol uptake of lesions with no residual hyperarterial tumor suggesting complete response
No comparison between absorbable and nonabsorbable particles has been made in LM from NETs. Moreover, most studies have included patients treated with absorbable and nonabsorbable particles [27, 30, 35, 36] (Fig. 26.3). Only one study has focused on TAE with trisacryl gelatin microspheres (Embosphere®) (Fig. 26.4). Hepatic embolization was performed using either particles sized 300–500 μm, 500–700 μm, and/or 700–900 μm. Absence of disease progression was seen in 91 % of the cases, and 35 % of the patients had partial response on imaging using RECIST criteria despite the fact that some patients had extensive tumor necrosis [19]. No major complications occurred in this series. Notably, all patients with bilobar involvement were treated sequentially [19].
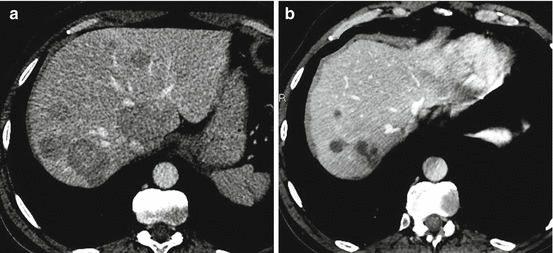
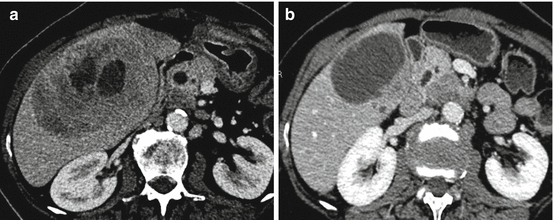

Fig. 26.3
A 56-year-old man with resected small bowel neuroendocrine tumor. (a) Axial CT scan shows hypoattenuating liver metastases (portal venous phase). (b) Axial CT scan at the portal phase after bland embolization of the right liver with sponge shows partial response with important necrosis and decrease in size of all lesions

Fig. 26.4
A 67-year-old woman with resected small bowel neuroendocrine tumor. (a) Axial CT scan shows big hypoattenuating liver metastases with little area of necrosis (portal venous phase). (b) Axial CT scan at the portal venous phase after bland-selective embolization of this lesion with microspheres shows a possible complete response with major necrosis and decrease in size of the lesion
Studies have compared the conventional TACE technique and the drug-eluting beads technique and have shown a more prolonged retention of drug within hepatocellular carcinoma in the latter [37]. Drug-eluting beads are particles which are preloaded with any chemotherapeutic agent. The principle is to deliver high dose and more sustained release of drug into the tumor compared to systemic chemotherapy [38, 39]. Recently, three trials have evaluated drug-eluting beads with doxorubicin in LM from NETs. Preloaded in LM from neuroendocrine tumors is doxorubicin (DC Bead, Terumo, Japan) [38, 39]. Stabilization or partial response on imaging was observed in 95 and 100 % of cases. The mean PFS was 14 and 15 months, respectively [38, 39]. Again, no comparison has been made with conventional TACE in those patients. Yet, the PFS rates were in the range of the others. Interestingly, biliary and liver injuries such as dilated bile ducts, portal vein narrowing, portal venous thrombosis, and biloma/liver infarcts have been reported in patients with LM from neuroendocrine tumors and are more often observed than in patients with hepatocellular developed on cirrhosis [40]. This first observation was largely confirmed by a study which showed that 7/13 (54 %) patients with LM from neuroendocrine tumors developed bilomas which forced interruption of the trial. Notably, all of these patients had multiple small LM [41]. It is hypothesized that hypertrophied peribiliary plexus observed in cirrhosis could protect against the ischemic/chemical insult of bile ducts suggesting caution when using drug-eluting beads in noncirrhotic liver [40].
26.3.3 Complications, Toxicity, and Exclusion Criteria
In a retrospective series of 72 patients with neuroendocrine LM, the median length of stay was 4 days [42]. The most common and classical complication is the postembolization syndrome which is seen in up to 80–90 % of the patients [27, 43]. It includes fever, leukocytosis, abdominal pain, nausea, and a transient increase in liver enzymes. Some of the severe complications are also observed in other liver malignancies such as liver failure, cholecystitis, gastric ulcers and bleeding, whereas some others such as carcinoid crisis are specific of LM from neuroendocrine origin [5].
TAE and TACE can be repeated safely in patients with LM from neuroendocrine tumors and especially in patients with disease progression [44]. The complication rate after repeat TACE is lower than after the first TACE [44]. As in other indications, TACE used to be performed at fixed delays whatever the tumor response. The trend is now to adapt the number of sessions and the interval between sessions to the tumor response.
Portal vein thrombosis and hepatic insufficiency are considered exclusion criteria for both TAE and TACE [1]. As the odds ratio of developing abscess in patients with bilioenteric anastomosis is very high (894), TACE should be avoided in those patients [45]. In a retrospective series of 489 TACE performed in various tumors, the three patients who developed abscess formation had a neuroendocrine tumor and a bilioenteric anastomosis [46]; in another retrospective series, 48 % of patients (12/25) with bilioenteric anastomosis developed an abscess, and two of them died [47]. If it must be performed, very broad-spectrum prophylactic antibiotics and bowel preparation before the procedure should be considered [45].
Tumoral liver involvement is an important issue for both efficacy and toxicity. Best morphological responses are obtained in patients with limited liver involvement (<30 or <50 %) [22, 26]. On the other hand, toxicity is increased in major liver involvement (>70 or 75 %) [22, 26, 27, 48]. This threshold has been first used as an exclusion criterion by many teams. However, Gupta et al. have been able to treat many patients with >75 % liver involvement successfully and safely by treating only a small portion of the liver in each embolization session [27].
26.4 Radioembolization
Radioembolization is defined as the injection of micron-sized embolic particles loaded with radioisotope by use of percutaneous transarterial techniques. Radioembolization with yttrium-90 microspheres involves infusion of embolic microparticles of glass or resin impregnated with the isotope yttrium-90 through a catheter directly into the hepatic arteries. Yttrium-90 is a pure β-emitter and decays to stable Zr-90 with a physical half-life of 64.1 h.
The efficacy of this radioembolization technique, as for the chemoembolization, is based on the fact that intrahepatic malignancies derive their blood supply almost entirely from the hepatic artery, as opposed to the normal liver, which mainly depends on the portal vein. The microspheres are injected selectively into the proper hepatic artery and subsequently become lodged in the microvasculature surrounding the tumor. Very high irradiation doses are delivered to the tumors, whereas the surrounding liver parenchyma is largely spared. Sommer et al. have shown that the only baseline imaging parameter statistically associated with the progression-free survival (PFS) was the hypervascular pattern [49].
The use of yttrium-90 for the treatment of primary and secondary liver malignancies is no longer investigational or experimental, and both devices have got FDA and European approval.
The technique comprises two steps: The first step is patient eligibility and conditioning. Selective mesenteric and hepatic angiography and scintigraphy are performed to isolate the hepatic circulation by occluding extrahepatic vessels with prophylactic embolization of extrahepatic arteries (e.g., right gastric, gastroduodenal artery). The second step is the radioembolization therapy itself. Several days after patient eligibility and conditioning, treatment is performed with microsphere infusion proceeding at flow rates similar to that of the native hepatic artery. Treatment of the contralateral lobe, if needed, is usually performed 30–60 days.
The largest series of selective interval radiation therapy (SIRT) of LM from NET is a retrospective review of 148 patients from ten institutions. Complete and partial tumor response were seen in 2.7 and 60.5 % of the cases according to RECIST criteria, respectively [50]. Stable disease was observed in 22.7 % of the cases, and progressive disease occurred in only 4.9 % of the cases [50]. Similar results were reported in the other series including a prospective one [51–56]. Paprottka et al. have observed that 97.5 % of liver metastases become necrotic or hypovascular explaining the high rate of overall response when using imaging criteria which aim to depict tumor changes such as EASL or mRECIST criteria [54]. For Ceelen et al., the postprocedural MR parameters associated with a longer PFS were a both decrease in sum of diameters and arterial enhancement and an increase in necrosis [57].
Symptomatic responses were observed in 55–100 % [53, 58, 59]. Disease control rate was 93 %, much better than for other types of malignancy (59 % for colorectal primaries and 63 % for other primaries) [60].
Low toxicity is another advantage of radioembolization. Side effects are mainly represented by fatigue, nausea or vomiting, and abdominal pain; no Grade 4 toxicities but one were seen in articles which detail complications after the procedure. Moreover, no radiation-induced liver failure was described in those patients [50, 54, 58].
26.4.1 Embolization and Chemoembolization vs. Radioembolization
To date, there has been no randomized trial but two review papers and a multicenter, prospective treatment registry with radioembolization which have evaluated the efficacy of radioembolization and TAE/TACE in neuroendocrine LM [61–63]. Treatment efficacy seems similar. Time to progression (TTP) was not different from the groups [61]. TAE/TACE seems more appropriate in patients with bulky and large tumors which require a segmental targeted approach whereas radioembolization could be more advantageous in patients with small LM that have a miliary bilobar distribution.
26.5 Indications of Liver-Directed Treatments
Presence of liver metastases largely influences prognosis in all types of neuroendocrine tumors [64]. Prognosis has improved with significant overall survival increasing in both patients with LM from the jejunum/ileum and the pancreas undergoing multidisciplinary treatment [64]. This includes hepatobiliary surgery, locoregional, and/or medical therapies.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






