Magnetic Resonance Spectroscopy
Ognen A. C. Petroff
John S. Duncan
Introduction
In this chapter we review the application of magnetic resonance spectroscopy (MRS) to the investigation of the epilepsies in humans from both research and clinical perspectives. Initially we deal with proton MRS of the major metabolites that are detectable in vivo in focal and generalized epilepsies. We then consider the measurement of glutamate, glutamine, and γ-aminobutyric acid (GABA). Finally, we address the use of phosphorus MRS interictally and postictally and the effect of therapies on the spectra.
Hydrogen (Proton) Magnetic Resonance Spectroscopy
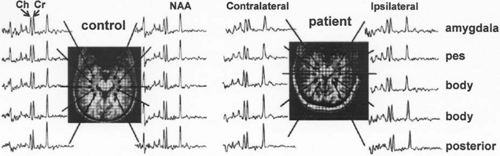
The principal signals, measured using long-echo-time (TE) proton (1H) magnetic resonance spectroscopy, include the singlet resonances of N-acetyl aspartate (NAA) (2.01 parts per million [ppm]), creatine plus phosphocreatine (CR; 3.0 and 3.9 ppm, respectively), choline-containing compounds (Cho; 3.2 ppm), and lactate (Lac; doublet signal centered at 1.35 ppm). The long TE suppresses signals from most other brain metabolites and macromolecules (lipids, proteins, and glycoproteins). N-acetyl-aspartate is the most concentrated organic metabolite unique to the central nervous system (CNS). The singlet resonance from the three hydrogen atoms of the terminal methyl group of the N-acetyl portion of NAA serves as a useful marker of normal adult nervous tissue (Fig. 1). The epileptic state appears to be characterized by a widespread though modest decrease in NAA, which is centered on the seizure onset zones in the focal epilepsies. Paradoxically, the loss of neurons in the epileptogenic focus exceeds the modest decrease in NAA, which suggests that NAA levels are above normal in the remaining cells, predominantly glia, but the area of NAA reduction may be more extensive.
The Acute Effects of Seizures
Relatively few patients have been studied during or soon after seizures using MRS. A recent review has summarized these case reports involving patients with partial status epilepticus.15 Lactate levels were increased and NAA levels reduced in the acute phase of the seizure. The difficulty in interpretation lies in distinguishing the contribution of neuronal injury from the metabolic effects of the prolonged seizure. Two epilepsy centers reported MRS studies of patients measured soon after mesial temporal lobe seizures.18,19 Lactate levels were elevated in the epileptogenic mesial temporal lobe obtained during complex partial seizures or within 4 to 24 hours after the seizures. Although NAA levels were below normal in all patients, there was no difference in NAA levels among serial spectra taken in the ictal, postictal, and interictal states. Generalized seizures were not associated with raised ictal lactate levels or decreased NAA levels. A significant decrease in the magnetization transfer ratio (MTR) of Cho was seen after seizures. Reduced MTR of Cho indicates a shift from a bound to a more mobile fraction. These changes might indicate membrane perturbation in areas of seizure spread.44
N-Acetyl Aspartate Levels of the Temporal Lobe Epilepsies
Several series of 30 to 100 patients with temporal lobe epilepsy (TLE) report that NAA and NAA/Cr ratios are decreased in the epileptogenic temporal lobe.17,21,28,46,57,122 In TLE, low NAA levels are often widespread and can affect the temporal lobe contralateral to the epileptogenic hippocampus and correlate with interictal hypometabolism measured using positron emission tomography (PET).80,96,102,113 The widespread changes seen using PET and MRS are attributed to diaschisis. Low NAA levels in the contralateral temporal lobe often herald a poor outcome from surgery in terms of seizures and cognitive function.47,92 Below-normal NAA levels can provide evidence of temporal lobe or hippocampal abnormalities in patients with TLE who show no abnormality on magnetic resonance imaging (MRI).29,111,167,183 Lesser decreases in NAA levels are seen in the temporal lobe contralateral to the epileptogenic one. About half of the patients with newly diagnosed TLE and unremarkable MRI have low NAA/Cr ratios.97 There may be subtle decreases in neuronal density in a hippocampus that appears normal on MRI.33
What Is the Impact of Seizure Frequency on N-Acetyl Aspartate Levels in Temporal Lobe Epilepsy?
The NAA/Cr ratios of the mesial temporal lobe ipsilateral to the seizure focus correlated with seizure frequency but not with the duration of the epilepsy.50 Similar findings were reported for frontal lobe epilepsy. Mesial temporal NAA levels and the NAA/(choline plus creatine) ratios were normal in patients with neocortical epilepsy, from which the authors inferred that repeated neocortical seizures did not cause secondary damage to the hippocampus.185 However, 5 of 10 patients with neocortical epilepsies had low NAA/(creatine plus choline) ratio in the mesial temporal lobe ipsilateral to the seizure focus, which suggests that seizure activity arising elsewhere in the same cerebral hemisphere may reduce hippocampal NAA levels and, by inference, neuronal function.
What Is the Impact of Interictal Epileptiform Discharges on N-Acetyl Aspartate Levels?
There have been several correlations of MR spectroscopic imaging (MRSI) with interictal epileptiform discharges (IEDs). In 9 patients with TLE and five with frontal lobe epilepsy there was a negative association between IED frequency and the decrease in NAA/Cr ratios, and the NAA/Cr ratio of the voxels nearest to the surface electrode with the highest IED frequency tended to be lowest.159 In 31 patients with TLE there was a high degree of concordance between unilateral IED and the number of voxels with below-normal NAA/Cr ratios and a moderate concordance between the distribution of NAA abnormalities and the field of the IED.106 In patients with lateralized NAA abnormalities and lateralized ictal EEG onset, however, the data were discordant in 6 of 22 (27%). In 32 patients with mesial temporal sclerosis (MTS), the NAA/Cr ratios in the ipsilateral medial temporal lobe were significantly lower than in the contralateral side and NAA/Cr ratios of both mesial temporal lobes. There was no significant correlation between the NAA/Cr ratio and IED frequency on the side with MTS, but there was a significant inverse correlation between IED and NAA/Cr contralaterally. The clinical significance of these data are unclear, but they suggest a relationship between IED and lower NAA levels in hippocampi that appear normal on MRI.123 Alterations in NAA levels also were reported in focal areas of IED, which were identified by magnetoencephalography (MEG), in nonlesional temporal lobe epilepsy.163 The NAA/choline ratios were decreased significantly in the spike zone compared with the contralateral homologous region but did not correlate with IED frequency.
Do N-Acetyl Aspartate Levels Progressively Decline in Temporal Lobe Epilepsy?
NAA/CR ratios were lower with longer duration of epilepsy in refractory TLE, both ipsilateral and contralateral to the epileptogenic temporal lobe.10,34,176 Declining NAA levels ipsilateral to the seizure focus may be attributed to neuronal loss. The decline in NAA/Cr ratios in the contralateral mesial temporal lobe is not as easily attributed to neuronal loss alone. Axonal injury can contribute to decreased NAA levels in the absence of neuronal loss.45,145 Frequent seizures spreading to the contralateral hippocampus potentially could reduce NAA levels in the contralateral hippocampus. Patients with TLE and a history of secondarily generalized tonic–clonic seizures are more likely to have bilaterally low NAA levels than are patients who have infrequent generalized seizures.92 Other, smaller studies have not found a significant relationship between duration of the epilepsy and NAA levels.16,37,185 Several groups have reported further, age-related decreases in hippocampal NAA levels in healthy volunteers, and so inclusion of an age-matched control group is essential to interpreting findings in those with epilepsy.175
N-Acetyl Aspartate Levels and the Pathology of the Epileptogenic Hippocampus
It is not clear whether decreased NAA levels in the mesial temporal lobe reflect hippocampal neuron loss. Initial studies attributed the low NAA/Cr ratio of the epileptogenic temporal lobe to neuronal loss and the accompanying loss of neuropil and axons.65 A study of the epileptogenic human hippocampus resected at surgery reports that NAA content is significantly lower in “sclerotic gliotic” specimens than those that are “histologically unremarkable.”126 Another semiquantitative study suggested that there is an association between low NAA levels measured in vivo with MRS and the severity of hippocampal sclerosis.34 There was no association between the hippocampal Cr/NAA ratios measured in vivo by MRS and neuron/glia ratios of the portion of the resected hippocampi.87 It was suggested that metabolic alterations associated with seizure activity contributes as much as altered neuron/glia ratios to the NAA/Cr ratio. Hippocampi with severe neuronal loss and gliosis had the same or higher NAA levels as did hippocampi with little neuron loss. This would appear to suggest that NAA levels, although lower than normal, are much higher in hippocampi in MTS than would be anticipated from nonepileptogenic hippocampi with similar degrees of neuronal loss, for example, Alzheimer disease or stroke. A recent surgical series found no significant associations between hippocampal neuron loss and the cellular content of NAA despite a more than a threefold difference in neuron loss and a twofold increase in glial density.140,142 The modest decrease in NAA levels and the NAA/Cr ratios of the hippocampi with >70% neuron loss and a doubling of glial density were unexpected. The NAA
content, measured in hippocampal sclerosis with TLE, was too high for the degree of neuron loss with the attendant loss of NAA synthetase expression. In the adult nervous system, NAA appears to be localized to neuronal cytosol; it would appear to be unlikely that the intracellular NAA content is increased two- to-threefold in the remaining neurons of the most gliotic hippocampi. A more probable explanation is that the glial cells contain significant amounts of NAA. One hypothesis is that NAA is synthesized by glial precursor cells or abnormal glia. Enzyme and transporter expression, function, and localization are altered in the epileptogenic human hippocampus with sclerosis. In cell cultures, the NAA and total creatine content of O2A-oligodendrocyte precursor cells are higher than in cerebellar neurons.178 A recent study shows that “mature oligodendrocytes” grown in cell culture with ciliary neurotrophic factor (CNTF) contain high levels of intracellular total creatine and NAA, comparable to levels reported in a variety of neuronal cultures.13
content, measured in hippocampal sclerosis with TLE, was too high for the degree of neuron loss with the attendant loss of NAA synthetase expression. In the adult nervous system, NAA appears to be localized to neuronal cytosol; it would appear to be unlikely that the intracellular NAA content is increased two- to-threefold in the remaining neurons of the most gliotic hippocampi. A more probable explanation is that the glial cells contain significant amounts of NAA. One hypothesis is that NAA is synthesized by glial precursor cells or abnormal glia. Enzyme and transporter expression, function, and localization are altered in the epileptogenic human hippocampus with sclerosis. In cell cultures, the NAA and total creatine content of O2A-oligodendrocyte precursor cells are higher than in cerebellar neurons.178 A recent study shows that “mature oligodendrocytes” grown in cell culture with ciliary neurotrophic factor (CNTF) contain high levels of intracellular total creatine and NAA, comparable to levels reported in a variety of neuronal cultures.13
Spectroscopic imaging at 4 T before surgery found that NAA/CR ratios decrease from 78% of normal values in the posterior hippocampus to a nadir of 68% of normal in the anterior epileptogenic hippocampus in patients with MTS.61 The contralateral hippocampus also had lower NAA/CR ratios ranging from 80% of normal in the posterior hippocampus to 86% to 87% of normal in the anterior hippocampus. There was no significant correlation between NAA/Cr in the epileptogenic hippocampus, measured in vivo before surgery, and neuronal counts of the resected hippocampus. There was, however, a significant correlation between NAA/Cr and reactive glial counts averaged over CA1-CA4 and the dentate. There was no significant correlation between glial fibrillary acidic protein (GFAP) staining and neuronal counts. This suggests that in TLE, reductions in the hippocampal NAA/Cr ratios inversely correlate with the presence of reactive astrocytes, an accepted marker for injury, rather than with the loss of neurons, and that reactive glia are able to metabolize NAA to some degree. An alternative hypothesis is that there is a pool of NAA in astrocytes with a very slow turnover rate and both oligodendrocytes and astrocytes avidly take up NAA.
N-Acetyl Aspartate Levels Recover Following Successful Temporal Lobe Resection
In regions with low levels prior to surgery, NAA can recover to nearly normal values following successful temporal lobe resection.20,66,172,186 Most of the recovery occurs within 6 months and 95% by 2 years.160 The recovery of NAA following surgery indicates that neuronal loss probably is not the main cause of low NAA levels in the nonepileptogenic hippocampus. Seizure activity may inhibit mitochondrial synthesis of NAA; in addition, frequent seizures may enhance neuronal release of NAA with subsequent enhanced glial catabolism. It is not clear whether decreased NAA levels are an adaptive response to seizure activity, which may downregulate hippocampal excitability. Some investigators have suggested that improved NAA levels may contribute to improved cognitive functions with seizure freedom, perhaps through improved neuronal functioning.
N-Acetyl Aspartate Levels of the Nonlesional, Focal Neocortical Epilepsies
Nonlesional neocortical epilepsies are defined by a normal-appearing MRI; the epileptogenic region of the neocortex is defined usually by surface or intracranial video-electroencephalographic (EEG) monitoring. Spectroscopic imaging to measure NAA levels, NAA/Cr ratios, or NAA/choline ratios may be useful for localizing the epileptogenic zone.49,56,104,114,173 Findings in nonlesional neocortical epilepsies parallel those reported for nonlesional TLE. Seizure frequency correlates with the magnitude of the decrease in NAA/Cr ratios in the seizure focus in several studies. Decreased NAA levels also were seen in regions outside of the seizure onset zone in most patients, including the ipsilateral hippocampus in nearly half.56,92,114,173
N-Acetyl Aspartate Levels of Epileptogenic Focal Cortical Dysplasia
Several studies have measured interictal NAA levels or NAA/CR ratios of epileptogenic focal cortical dysplasia.85,104,187 Mean NAA levels of the dysplasia were lower by 17% to 27%, and the mean NAA/CR ratios decreased to 71% to 79% of normal. One study reported MRSI and quantitative cell counts for focal cortical dysplasia.85 The mean neuron-to-glia ratio was the same in the autopsy control and the patients. There was no evident correlation between the rate of IED and the NAA/CR ratios. However, the ratios were lower in patients with frequent seizures. The metabolic abnormalities closely correlated with the location of the epileptogenic focus among patients with focal cortical dysplasia with normal NAA/CR ratios in other cortical areas.
Spectroscopic Imaging of Heterotopia and Polymicrogyria
Malformations of cortical development (MCD) include diverse pathologies. The mean lesional NAA/CR ratios are variably altered in epileptic patients with heterotopia and polymicrogyria.95,115,195 There are many reports of no significant differences in the mean NAA/CR ratio between the heterotopia and the cortex or white matter of control sub-jects.85,116,192 Others have reported small decreases in the lesion.72,95,171,195 Similarly, there are reports of no significant metabolic alterations in the regions affected by polymicro-gyria,72,85,192 and others report significant decreases in the mean NAA/Cr ratio.171 MRSI studies have reported considerable heterogeneity within the volume involved with the MCD or the perilesional brain.95,115,195 NAA and creatine levels and their ratios may be decreased in one voxel within the lesions seen by MRI and increased in another voxel, with much of the lesion having normal levels. What is unclear is the relationship between these metabolic alterations and the seizure focus. Quantitative histopathology correlated to the metabolic measurements remains to be published. The clinical value of MRSI in addition to conventional MRI in this context remains to be assessed.
N-Acetyl Aspartate Levels in Idiopathic and Cryptogenic Epilepsies
Overall, the mean hippocampal NAA/CR ratio of children with rolandic epilepsy was increased by 12% to 14% compared with healthy children matched for age and development.103 However, lateralization of the lower NAA/CR ratios was concordant with the lateralization of the IED in 10 of 13 children. The mean NAA/CR ratio of the hippocampus contralateral to the IED was 20% above normal. The ipsilateral ratio was in the normal range (6% higher). In cryptogenic occipital lobe epilepsy, NAA
levels and NAA/CR ratios measured in the occipital lobe (61% cortical grey matter) were reported as normal.167 There have been several studies of idiopathic generalized epilepsies (IGEs), primarily of patients with juvenile myoclonic epilepsy (JME) or generalized tonic–clonic seizures (GTCS).11,19,110,155,156,169 Overall, the mean NAA/CR ratios have been about 10% below normal in the thalamus, 5% below normal in the frontal lobes, and within the normal range in the occipital lobes of those with idiopathic generalized epilepsies.
levels and NAA/CR ratios measured in the occipital lobe (61% cortical grey matter) were reported as normal.167 There have been several studies of idiopathic generalized epilepsies (IGEs), primarily of patients with juvenile myoclonic epilepsy (JME) or generalized tonic–clonic seizures (GTCS).11,19,110,155,156,169 Overall, the mean NAA/CR ratios have been about 10% below normal in the thalamus, 5% below normal in the frontal lobes, and within the normal range in the occipital lobes of those with idiopathic generalized epilepsies.
Summary on the Utility of N-Acetyl Aspartate Measurements in the Human Epilepsies
Measurements of NAA levels using MRSI appears to be most useful in focal epilepsies without lesions seen using conventional MRI. Low NAA levels and NAA/Cr ratios are widespread, with the lowest values associated with the areas most frequently and intensively involved by the seizures. NAA imaging may offer clues to the regions where the seizures start or those areas that facilitate the spread of the seizure to more normal cortex. Detailed NAA images may complement EEG- and MEG-based maps of epileptogenic networks. NAA images may help lateralize mesial TLE, but bilateral abnormalities are common. Based on the use of single voxels, the reported sensitivity of the NAA/CR ratio in determining the epileptogenic hemisphere was 60% to 65%.28,92 With the use of MRSI, the lateralization improves to 85% to 98%.17,21,83,86,98 More important, severe bilateral hippocampal involvement appears to be associated with less desirable surgical outcomes. Combined measurements of NAA/CR with MRSI and volumetric MRI correctly predicted the surgical outcomes of 39 of 52 (75%) patients with TLE who became seizure free and of 21 of 29 (72%) patients who did not.2 Combined measurements of the NAA/Cr ratio in the ipsilateral midtemporal region, asymmetry of the NAA/Cr ratio in the ipsilateral midtemporal region, ipsilateral hippocampal volume, and hippocampal asymmetry correctly predicted the surgical outcomes of 92% of patients who experienced a >90% reduction of seizure frequency and 63% of patients who did not. The biology of the lesion appears to interact with seizure frequency to affect NAA levels in focal cortical epilepsies with lesions evident by MRI. Further studies at higher field strength may offer the spatial resolution needed to distinguish the epileptogenic regions from the metabolic changes produced by the biology of the anatomic abnormalities. When there are multiple lesions, this approach may reveal the epileptogenic abnormalities. In patients with extensive cortical malformations, detailed NAA images may offer insights into the pathophysiology of these epilepsies. Overall, the spectroscopic imaging of NAA has not offered much insight into idiopathic generalized epilepsy. The interictal changes in NAA metabolism are small, with the greatest involvement localized to the thalamus.
Glutamate Metabolism
Glutamatergic neurons contain 80% to 88% of total brain glutamate, GABAergic neurons contain 2% to 10%, and astrocytes contain about 10%.68 Decreases in brain glutamate content, therefore, usually primarily reflect the loss of glutamatergic neurons and synapses.59 The glutamate content of human white matter is lower than that of gray matter.32,130 In normal brain, glutamate is known to be in high concentration in neurons, and astrocyte glutamate concentrations have been estimated in vivo to be comparatively low (<1 mM).118 The low intraglial glutamate concentrations are maintained primarily by the conversion of glutamate and ammonia into glutamine by glutamine synthetase.39 In addition, glutamate is oxidized by glutamate dehydrogenase (GDH) and enters the tricarboxylic acid (TCA) cycle. Neuronal glutamate is lost during glutamate transmitter release and is taken up by glia, where it is recycled by glutamine synthetase.31 Glutamate lost from the neuron is replaced through phosphate-activated glutaminase (PAG) acting on glutamine synthesized in the glia and transported into neurons.89,181 All glutaminases, including neuronal PAG, produce glutamate and ammonia. Ammonia is a mitochondrial toxin and is detoxified primarily by glial glutamine synthetase. If there is no neuronal loss, changes in tissue glutamate content reflect changes in neuronal glutaminase activity and indirectly the net synthesis of glutamate from glucose.
Proton Spectroscopy of Glutamate in the Human Epilepsies
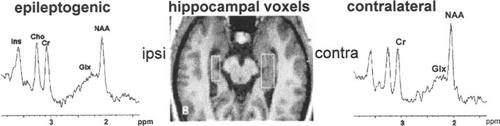
The signals from glutamate and glutamine are difficult to measure because of low signal-to-noise ratios and spectral crowding. Investigators using 1.5-T clinical spectrometers and short-TE pulse sequences have used the broad signals located between 2.1 to 2.5 ppm, which comprise resonances from glutamate, glutamine, NAA, various macromolecules, and, to a lesser extent, GABA, succinate, homocarnosine, glutathione, and N-acetylaspartylglutamate (NAAG), as a surrogate for glutamate plus glutamine (GLX) signals. Initial studies suggested that GLX might be useful in focal epilepsies with a normal MRI.154,194 GLX signals and the GLX/NAA ratios were reported to be above normal in the MRI-negative epileptogenic hippocampus (Fig. 2) or the epileptogenic areas of the nonlesional neocortical epilepsies. Subsequent studies reported greater variability of the GLX signals and GLX/NAA ratios, which were the same as or above normal values in various regions outside of the presumed seizure focus, that is, frontal lobes or contralateral hippocampus in TLE.44,167,190 The GLX signals showed considerable inter- and intrasubject variability in patients with MCD or Rasmussen encephalitis.191,195 Studies of patients with idiopathic generalized epilepsy suggest that the GLX signals and the GLX/NAA ratios of the frontal lobes are above normal.168,169 Stronger magnetic fields, reduced magnetic field inhomogeneity (shimming), better receiver design, and advanced pulse sequences facilitate the measurement of glutamate and glutamine by improving the signal-to-noise ratio, reducing spectral crowding, and ensuring a flat baseline.8,32,73,121,149
The glutamate content of gray matter, which contains a high density of synapses, is higher than values for white matter, which has less water content, more myelin, and few synapses.130 The glutamate concentrations of extracellular fluid (ECF) and cerebrospinal fluid (CSF) are 10,000-fold lower than neuronal intracellular values. Parsing each voxel into gray matter, white matter, and CSF (segmentation) helps to reduce variability in metabolite measurements.32,121 As expected, voxels that contain a greater fractional volume of gray matter have higher glutamate levels than those containing more white matter. In vivo measurements show that the glutamine content of white matter appears to be 50% to 60% of gray matter values. Biopsy material, however, has shown a uniform glutamine content between lateral temporal neocortex and subjacent white matter.
The Effects of Antiepileptic Drugs on Cortical Glutamate and Glutamine Levels
Antiepileptic drugs may contribute to the variability in GLX, glutamate, and glutamine levels in patients who take these
medications.131,134,136 Measurements made in the visual cortex of patients with refractory complex partial seizures, primarily temporal or frontal lobe epilepsies, suggest that glutamate levels are modestly but significantly increased in patients taking carbamazepine, phenytoin, or gabapentin and are low in patients taking phenobarbital or primidone. Whether barbiturates lower tissue glutamate content remains to be determined. Cortical glutamate content decreases by 16% to 28% with pentobarbital anesthesia.124,165 Valproate and vigabatrin increase human brain glutamine levels by 50% to 80%. Valproate increases blood ammonia levels through its effects on human kidney glutamine metabolism. Vigabatrin is an irreversible inhibitor of GABA-transaminase and may inhibit, albeit to a lesser degree, the other transaminases, including ornithine aminotransferase, thereby raising ammonia levels.62 Whether the use of valproate contributes to the increase of frontal lobe GLX levels in patients with idiopathic generalized epilepsy remains speculative.
medications.131,134,136 Measurements made in the visual cortex of patients with refractory complex partial seizures, primarily temporal or frontal lobe epilepsies, suggest that glutamate levels are modestly but significantly increased in patients taking carbamazepine, phenytoin, or gabapentin and are low in patients taking phenobarbital or primidone. Whether barbiturates lower tissue glutamate content remains to be determined. Cortical glutamate content decreases by 16% to 28% with pentobarbital anesthesia.124,165 Valproate and vigabatrin increase human brain glutamine levels by 50% to 80%. Valproate increases blood ammonia levels through its effects on human kidney glutamine metabolism. Vigabatrin is an irreversible inhibitor of GABA-transaminase and may inhibit, albeit to a lesser degree, the other transaminases, including ornithine aminotransferase, thereby raising ammonia levels.62 Whether the use of valproate contributes to the increase of frontal lobe GLX levels in patients with idiopathic generalized epilepsy remains speculative.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree