Chapter 110 Management of Obstructive Sleep Apnea–Hypopnea Syndrome
Abstract
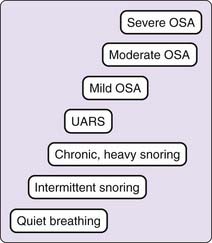
Sleep-related breathing disorders are common, are present in all age groups, and can result in significant morbidity and mortality. Eighty percent of patients presenting to sleep disorders centers have a manifestation of sleep-disordered breathing as the primary diagnosis. The term sleep-disordered breathing has been used synonymously with the term obstructive sleep apnea (OSA) syndrome, which has been supplanted by the term obstructive sleep apnea–hypopnea (OSAH) syndrome. In this chapter, we use the term sleep-disordered breathing (SDB) to encompass the entire gamut of respiratory abnormalities occurring during sleep. We will apply the term obstructive sleep apnea–hypopnea (OSAH) syndrome in reference to the spectrum of disorders in the family of obstructive respiratory events, including obstructive sleep apnea (Fig. 110-1).
This chapter presents an overview of the identification and management of patients with SDB. Details of diagnosis, clinical features, pathophysiology, and treatment are presented in Chapters 101 to 109.
Epidemiology and Risk Factors
Prevalence and Incidence in the General Population
The Wisconsin Sleep Cohort Study reported that 4% of men and 2% of women in a middle-aged cohort (ages 30 to 60 years) had obstructive sleep apnea, defined as an apnea–hypopnea index (AHI: the average number of apneas plus hypopneas per hour of sleep) greater than 5, and also daytime hypersomnolence.1 In this cohort, 44% of men and 28% of women were habitual snorers. Since this report in 1993, the general population has become heavier and older. The risk for significant SDB rises both body mass index (BMI: weight in kilograms per height in meters squared)2 and with age.3
It is difficult to identify a precise prevalence for sleep apnea for two reasons: Risk factors in the general population are increasing, and the diagnostic criteria continue to evolve as more is learned about physiologic and clinical outcomes. However, the Sleep Heart Health Study (SHHS) reported that 22% of 1824 people had a respiratory disturbance index (RDI) of greater than 15 events per hour. In the context of this particular research study, the RDI was synonymous with the AHI, and the presence or absence of symptoms was not considered in this assessment of prevalence. Most practitioners would consider an RDI greater than 15 to be significant OSAH. However, the SHHS cohort was not representative of the general population because it was enriched with snorers.4 A National Sleep Foundation Poll5 found that about a third of middle-aged adults had signs and symptoms suggesting sleep apnea.5 Certain groups, such as commercial vehicle drivers, have consistently been shown to have a very high prevalence of significant SDB.6,7 The high rates of obesity, male gender, cigarette smoking, and older age in commercial drivers are among the reasons that this particular group is at increased risk for OSAHS.
On the basis of an exhaustive review of currently available data, it has been conservatively estimated that 5% of adults have OSAH with sleepiness, and an unknown percentage have SDB without overt sleepiness.8
The Cleveland Family Study of 285 persons without significant sleep apnea at baseline reported that the incidence of developing SDB (as defined by an AHI > 5 events per hour) is about 7% per year, and the incidence of developing an AHI of greater than 15 events per hour is about 2% per year.9 In addition to establishing the first incidence data for SDB, this study confirmed that with aging, male sex and BMI lose importance as risk factors for obstructive sleep apnea.3,9 After menopause, the incidence of SDB rises in women, and the prevalence difference between the sexes essentially vanishes.10
Genetic Influences
Sleep-disordered breathing is seen more commonly in patients with a family history of SDB (see Video 103-1![]() ). In one study, 41% of the offspring of 45 randomly selected patients with OSAH syndrome had an AHI greater than 5, and 13.3% had an AHI greater than 20.11 Other large family studies have reported a much higher prevalence of OSAH syndrome among offspring of family members with OSAH when compared to the general population (see Chapter 103).8 Ethnicity also appears to play a role in the development of SDB. African Americans, Asians, and Latin Americans are at increased risk for sleep apnea, even controlling for other important risk factors such as BMI.2,8,12,13
). In one study, 41% of the offspring of 45 randomly selected patients with OSAH syndrome had an AHI greater than 5, and 13.3% had an AHI greater than 20.11 Other large family studies have reported a much higher prevalence of OSAH syndrome among offspring of family members with OSAH when compared to the general population (see Chapter 103).8 Ethnicity also appears to play a role in the development of SDB. African Americans, Asians, and Latin Americans are at increased risk for sleep apnea, even controlling for other important risk factors such as BMI.2,8,12,13
Associated Medical Disorders
Although it was recognized long ago that heart failure can result in repetitive central apneas (Cheyne-Stokes breathing—see Chapter 100), it is now clear that OSAH can contribute to left ventricular dysfunction and exacerbate congestive heart failure. Thus, SDB should be suspected in all cases of heart failure.14 OSAH also appears to be an independent risk factor for atrial fibrillation15 and stroke.16 Hypothyroidism is more prevalent in those with OSAH than in those without, and it can contribute to the development of SDB by several mechanisms, including obesity, increased tongue size, and reduced ventilatory drive.8,17 Nasal obstruction and rhinitis are also associated with increased snoring and SDB; unfortunately, however, surgery directed exclusively at the nose has a low therapeutic yield.18 Enlarged tonsils and adenoids can cause OSAH, particularly in children. OSAH should be strongly suspected in syndromes that are associated with altered craniofacial morphology, upper airway soft tissue anatomy, and airway caliber such as trisomy 21, achondroplasia, Arnold-Chiari malformation, mucopolysaccharidoses, and Klippel-Feil, Pierre Robin, Alpert, Treacher Collins, and Marfan syndromes.8
Behavioral Factors
Alcohol and sedative medications decrease neuromuscular drive to the upper airway dilator muscles, predisposing to recurrent upper airway collapse.8,19 Tobacco smoke causes an increase in nasal and pharyngeal irritation, resulting in narrowing of the upper airway. OSAH syndrome has been shown to be more prevalent in current smokers than in nonsmokers or ex-smokers.8,20–22
Pathogenesis
Epidemiologic and genetic studies indicate that the inheritance of OSAHS is likely to be complex, and its development depends on environmental as well as genetic factors (see Chapter 103). Obesity increases the inspiratory work of breathing because the effort of displacing central obesity results in abnormally high levels of negative pressure (suction) against pliable tissues of the posterior pharyngeal space. Suction on the pliable, soft tissues of the upper airway can result in upper airway edema, which is exacerbated by the vibratory trauma of snoring. If there is nasal obstruction (polyps, septal deviation), then increased upstream airway resistance increases the effects of the increased downstream negative pressure (intrathoracic suction). A simple analogy is the collapse of a paper straw that is partially crimped along its length when a very strong suction is applied at one end. For more on the pathophysiology of sleep-disordered breathing, see Chapters 101 and 102. Several studies have also demonstrated that closure or narrowing of the upper airway occurs during inspiration in the breaths before obstructive events.23
Clinical Features
History
Snoring
Heavy snoring is the most common symptom in patients with SDB (Box 110-1, Videos 110-1, 110-2, and 110-3![]() ). About 50% of men and 25% of women snore, but somewhat fewer than that have OSAH, so snoring alone is clearly not diagnostic. Snoring accompanied by a bed partner’s reports of observed apnea, snorting, gasping, and choking during sleep is predictive of OSAH.24–26 Witnessed apneas are more predictive of SDB than are patient-reported episodes of waking up gasping for breath, which may be a symptom of other diseases such as congestive heart failure, gastroesophageal reflux disease, nocturnal asthma, and panic disorder. Nonetheless, a patient’s complaints of awakening with sensations of gasping or shortness of breath should be explored.
). About 50% of men and 25% of women snore, but somewhat fewer than that have OSAH, so snoring alone is clearly not diagnostic. Snoring accompanied by a bed partner’s reports of observed apnea, snorting, gasping, and choking during sleep is predictive of OSAH.24–26 Witnessed apneas are more predictive of SDB than are patient-reported episodes of waking up gasping for breath, which may be a symptom of other diseases such as congestive heart failure, gastroesophageal reflux disease, nocturnal asthma, and panic disorder. Nonetheless, a patient’s complaints of awakening with sensations of gasping or shortness of breath should be explored.
Sleepiness
Although excessive daytime sleepiness has many possible etiologies, this complaint should increase the suspicion for OSAH syndrome. Subjective sleepiness can be assessed by the Epworth Sleepiness Scale (see Chapter 143, which has been validated in clinical studies and correlates very roughly with objective measures of sleepiness.27 Patients underreport and overreport their sleepiness,28 so querying members of their household is also useful. An Epworth Sleepiness Scale score greater than 10 suggests significant daytime sleepiness, but this is not specific for OSAH syndrome. Sleepiness can be objectively measured with a multiple sleep latency test or with the maintenance of wakefulness test, but these tests are rarely indicated in the routine evaluation of persons with suspected or diagnosed OSAH syndrome. Patients who report falling asleep while driving or performing other safety-sensitive tasks should be evaluated for a variety of sleep disorders, including SDB (see Video 104-1![]() ). Because of the threat posed to the patient and others by this symptom, clinicians should have a low threshold for evaluation of patients who report this problem.
). Because of the threat posed to the patient and others by this symptom, clinicians should have a low threshold for evaluation of patients who report this problem.
Gender and Symptoms
Female patients with OSAH are much more likely than male patients to present with insomnia, and they are less likely to present with a history of observed apnea. Women with OSAH are much more likely than men to have a diagnosed mood disorder and hypothyroidism and are more likely than men to report symptoms of restless legs, nightmares, palpitations, and hallucinations.34
Alcohol and caffeine have been found to be used more by male than by female patients.
Physical Findings
The physical finding that is most predictive of OSAH syndrome is central obesity. BMI is a convenient but imperfect surrogate for central obesity. A BMI greater than 28 kg/m2 in both men and women reflects a risk factor and should increase the suspicion for OSAH syndrome. Approximately 40% of persons with a BMI greater than 40 and 50% of those with a BMI greater than 50 have significant SDB.2 Premenopausal women with OSAH are generally much heavier than their male counterparts, and both obesity and sex become less important risk factors for OSAH after age 50 years.8
Measures of central obesity such as neck size are also very useful in predicting the presence of OSAH. Men with a neck circumference greater than 17 inches (43 cm) and women with a neck circumference greater than 16 inches (41 cm) are at particular increased risk for OSAH syndrome confirmed by overnight polysomnography.25,35,36 Nasal obstruction from any cause appears to be a risk factor for SDB, including snoring.37 Several diagnostic schemes are based on intraoral measurements, notably a narrowed posterior oropharynx.26,37,38
Measurement of systemic arterial blood pressure is, of course, a standard part of the physical examination. It is therefore noteworthy that several large epidemiologic studies have demonstrated that OSAHS is a risk factor for hypertension39–41 in a dose-dependent way. Analysis of data obtained from 6132 subjects in the SHHS revealed an odds ratio for hypertension of 1.37 (95% confidence interval [CI], 1.03 to 1.83), comparing the highest category of AHI (>30/hr) with the lowest (<1.5/hr),39 even after adjustment for other factors including age and BMI. In one small study, 83% of patients with drug-resistant hypertension had OSAH.42 Thus, the presence of hypertension, particularly difficult-to-control hypertension, should increase the clinical suspicion for OSAH.
Diagnosis
Polysomnography
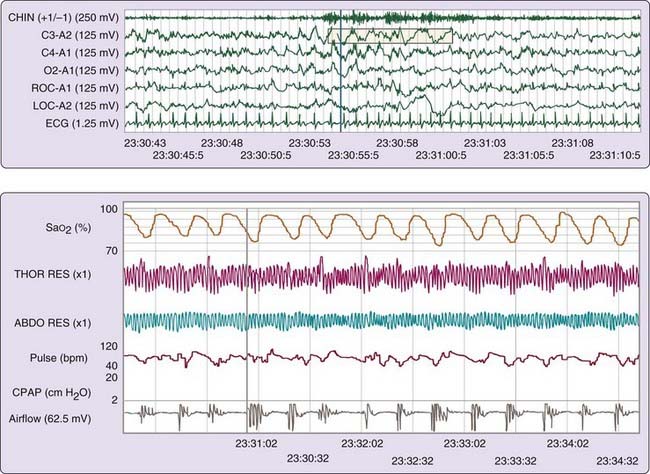
The gold standard for the diagnosis of OSAH remains overnight polysomnography (PSG) (Fig. 110-2). A nocturnal PSG includes recordings of airflow, ventilatory effort, oxygen saturation, and body position, as well as electrocardiography, electromyography, and electroencephalography. In standard, laboratory-based PSG, a technician is present to monitor the patient during the entire study. A single overnight PSG is generally sufficient to diagnose OSAH, but it might not be sufficient to rule it out, particularly if the SDB is caused or exacerbated by factors not present during the study, such as rapid eye movement (REM) sleep, supine sleep, or alcohol ingestion. In many instances, the level of SDB is severe enough that the diagnosis of OSAH can be established early in the study. In this event, a split-night study may be performed, in which the second half of the study is used to titrate treatment (positive airway pressure) for OSAH.
Definitions
The AHI is the most commonly used criterion to establish the diagnosis of OSAH and to quantify its severity. The AHI is defined as the total number of apneas plus hypopneas divided by the hours of sleep (Box 110-2) in a single night’s study. Obstructive apneas and hypopneas are characterized by an absence or a reduction of airflow, respectively, despite continued inspiratory efforts. Some authors have suggested that the presence of continuous positive airway pressure (CPAP) responsiveness might add additional precision to the diagnosis.43 In central apneas and hypopneas, inspiratory effort is absent or reduced during the period of apnea or reduced airflow.
Box 110-2 Definitions
Apnea
Hypopnea
The American Academy of Sleep Medicine (AASM) has recently published a revised scoring manual for polysomnography.44 In this manual, apnea is defined as a drop in the peak thermal sensor (thermocouple) excursion by >90% of baseline, lasting at least 10 seconds, with at least 90% of the event meeting the amplitude-reduction criterion. Apneas are obstructive if there is continued or increased inspiratory effort, and they are central if there is not. The designation of mixed apnea is retained; mixed apnea is described as absent effort at the beginning, with resumption of effort before resumption of airflow.
It is useful to consider these definitions in light of the Sleep Heart Health Study (SHHS), which is a longitudinal follow-up of more than 6000 persons including in-home PSG.45 In this study, limited to middle-aged and older adults, apneas were identified by absence of airflow for greater than 10 seconds, and hypopneas were defined by a reduction of airflow (to 30% of baseline for apneas and to 70% of baseline for hypopneas) for longer than 10 seconds. In the SHHS, the definitions of apneas and hypopneas require an oxygen desaturation of 4% or more, and they do not include any measure of sleep disturbance or arousal.45–47 These criteria were adopted largely because they are both predictive of cardiovascular sequelae47 and are very reproducible.45,46
Thus, there is a slight discrepancy between the definition of apnea proposed by the American Academy of Sleep Medicine (AASM),44 which does not require oxygen desaturation, and that used in the SHHS, which does.
A careful retrospective analysis of SHHS data has demonstrated that only the only definition of hypopneas that includes at least a 4% oxyhemoglobin desaturation is associated with prevalent cardiovascular disease.48 This is not to say that different desaturation criteria would not be more predictive of other outcomes, such has glucose intolerance.49
Diagnostic Criteria
Obstructive Sleep Apnea–Hypopnea Syndrome
According to the AASM, OSAH syndrome exists when a patient has five or more obstructed breathing events per hour of sleep, with an appropriate clinical presentation (Box 110-3).50 Centers for Medicare and Medicaid Services (CMS) reimburses for CPAP treatment for patients with an AHI greater than 15 or an AHI > 5, with associated hypertension, stroke, sleepiness, ischemic heart disease, or mood disorder (see below, page 1284, Indications).51
Box 110-3
Adapted from American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed. Diagnostic and coding manual. Westchester, Ill: American Academy of Sleep Medicine; 2005.
Obstructive Sleep Apnea, Adult
A, B, and D or C and D satisfy the criteria
Although there is now some consistency in the definitions of SDB, considerable variation continues to exist in recording techniques for measures of airflow and respiratory effort (see Chapter 142).50,52 The innate inaccuracy and variability of current measurement techniques have almost certainly resulted in varying sensitivities for detection of SDB events.
Upper Airway Resistance Syndrome
The original description of the upper airway resistance syndrome (UARS) emanated from careful study of a small group of persons who had some of the clinical features of OSAH syndrome but negative sleep studies.53 In these patients, increased respiratory effort was identified by esophageal pressure nadirs (measured by esophageal pressure manometry) more negative than one standard deviation below the mean, followed by transient electroencephalography (EEG) arousals. These events were termed respiratory effort–related arousals (RERAs), and patients who had five or more such events per hour slept and complaints of sleepiness were deemed to have UARS. Patients with UARS require increased inspiratory effort to generate inspiratory airflow, and this is associated with frequent arousals but without overt apneas (see Videos 102-1 to 102-4![]() ). This fragmented sleep is believed to result in increased subjective and objective daytime sleepiness.
). This fragmented sleep is believed to result in increased subjective and objective daytime sleepiness.
Much of the current confusion and controversy surrounding UARS probably reflects the lack of standardized definitions and recording techniques. An AASM panel defined a RERA event as “a sequence of breaths characterized by increasing respiratory effort leading to an arousal from sleep, but which does not meet criteria for an apnea or hypopnea.”50 The revised scoring manual also defines RERAs using nasal pressure (they were originally defined using esophageal pressure) as “a sequence of breaths lasting at least 10 seconds characterized by increasing respiratory effort or flattening of the nasal pressure waveform leading to an arousal from sleep when the sequence of breaths does not meet criteria for an apnea or hypopnea.” In other words, both the measurement techniques and the precise definition of RERAs remain somewhat ill-defined. However, a perhaps oversimplified definition of UARS might be five or more RERAs (however defined) per hour of sleep with daytime sleepiness.
Techniques that are currently used to detect RERAs in sleep laboratories vary greatly in clinical practice, but few clinical centers actually define and measure UARS as it was originally defined. Because there is no clear standard of diagnosis for this condition, it is probably both underdiagnosed and overdiagnosed (see Chapters 102 and 142).
Other Diagnostic Approaches
Prediction Formulas and Questionnaires
Because individual features from the history and physical diagnosis are often nondiagnostic, several groups have suggested the use of prediction formulas based on combinations of findings.24–2636 Among the most useful of such findings are history of witnessed apneas, male sex, BMI, and neck circumference. The Berlin Questionnaire25 focuses on a limited set of known risk factors for OSAH including questions about snoring, daytime sleepiness, and high blood pressure, as well as age, weight, height, sex, and neck circumference (see Chapters 105 and 142).
Specific oropharyngeal measurements are also highly predictive.26,38 Investigators of prediction formulas typically analyze the ability of the proposed formula to predict a given AHI. However, because the AHI does not take into account the degree or duration of oxygen desaturation, sleep disturbance, or cardiac arrhythmias, it is a suboptimal gold standard. A more clinically relevant outcome for prediction formulas might be a positive response to CPAP treatment.43 In general, these formulas perform well when applied to persons with a high likelihood of severe SDB, but this is when such formalized algorithms are least needed by the clinician. Prediction formulas probably have a place in the expedited diagnosis or triage of patients with severe OSAH, particularly in patients with typical presentations, such as sleepy, obese, hypertensive, middle-aged men.
Home Sleep Testing
Ambulatory Multichannel Studies
In the spring of 2008, the CMS announced its intent to pay for CPAP treatment on the basis of portable testing (home monitoring) and also announced that continued reimbursement for CPAP after the first 12 weeks of CPAP treatment will be contingent upon demonstration of benefit to the patient. The CMS statement also stipulated that patients should be evaluated by qualified clinicians.51 This decision came after a careful analysis of the available data, including an Agency for Health Quality (AHRQ) report that found that in-laboratory testing does not confer obvious benefit in patient outcomes compared with home testing.54 This national coverage decision (NCD) will be implemented regionally based on local coverage determinations (LCDs), and there is likely to be some regional variation in implementation and a gradual transition. Testing for sleep-disordered breathing is addressed in more detail in Chapter 142.
Controversy continues about where sleep studies are best done (Video 110-4![]() ) and about the role of screening in general.55,56 Screening has the potential to actually delay diagnosis and to result in false-negative results. In general, screening for OSAH may be useful to screen a person in but not out. Clearly, not all patients will be good candidates for home testing, and the currently proposed reimbursement level for home studies is unlikely to result in a rapid transition from in-laboratory to home testing. Theoretically, home monitoring may result in increased accessibility, enhanced patient convenience, reduced cost, and better sleep in the familiar environment. However, equipment problems cannot be corrected, non-OSAH disorders cannot be detected, and CPAP titrations cannot be performed with home testing. Because autotitrating CPAP performs at least as well as in-laboratory titrated CPAP in patients who have the classic presentation of OSAH syndrome (see later and Chapter 107), the last concern is not particularly valid.
) and about the role of screening in general.55,56 Screening has the potential to actually delay diagnosis and to result in false-negative results. In general, screening for OSAH may be useful to screen a person in but not out. Clearly, not all patients will be good candidates for home testing, and the currently proposed reimbursement level for home studies is unlikely to result in a rapid transition from in-laboratory to home testing. Theoretically, home monitoring may result in increased accessibility, enhanced patient convenience, reduced cost, and better sleep in the familiar environment. However, equipment problems cannot be corrected, non-OSAH disorders cannot be detected, and CPAP titrations cannot be performed with home testing. Because autotitrating CPAP performs at least as well as in-laboratory titrated CPAP in patients who have the classic presentation of OSAH syndrome (see later and Chapter 107), the last concern is not particularly valid.
The SHHS, using rigid protocols and a centralized PSG reading laboratory, demonstrated that home monitoring can produce reliable data with acceptable rates of data loss.45 The two nonapneic sleep disorders most likely to be identified by laboratory PSG are narcolepsy, which cannot be diagnosed by overnight study alone, and periodic limb movements during sleep. Periodic limb movements are extremely common in sleep disorders,57 often unassociated with sleepiness in the nonapneic patient,57 and in some patients may be a marker of SDB.58 A comparison of laboratory PSG and titration with home diagnosis and autotitration found no difference in CPAP compliance between groups, although the home testing group was evaluated more quickly and less expensively.59 Thus, although there are many unresolved issues about the large scale use of home sleep testing in the routine testing for OSAHS, CMS has clearly expanded the options for documenting OSAHS and starting CPAP treatment. At present, home sleep testing is most applicable to confirm the diagnosis of OSAH syndrome in persons with a high likelihood of the disorder.
Oximetry
Because the CMS NCD addressing CPAP treatment of OSAHS covers even the simplest (2-channel) home studies, some regions may stipulate that oximetry is sufficient to diagnose OSAH, so long as one other parameter (typically, heart rate) is also recorded. Oximetry results are the basis for the current definitions of SDB, in that oxygen desaturation of varying degrees are included or required criteria for measures of SDB, notably hypopneas.46,47,50–52
Oximetry has better interrater reliability, and it is a better predictor of the response to CPAP treatment than is the AHI.60,61 In general, patients with significant sleep apnea have a greater fluctuation in oxygen saturation (and heart rate) than do those without. However, thinner, younger patients without lung disease can have significant breathing and sleep disturbance without remarkable oxygen desaturation. Patients with underlying lung disease can have oxygen desaturation without OSA. Thus, oximetry is neither sensitive nor specific for SDB.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree