Chapter 106 Medical Therapy for Obstructive Sleep Apnea
Abstract
The principal therapy for obstructive sleep apnea (OSA) remains positive pressure delivered by a nasal or naso-oral interface (see Chapter 107). Oral appliances can also be useful in selected patients who cannot tolerate positive airway pressure (see Chapter 109). However, other medical options may be important as an adjunct to these treatment options or as a therapeutic intervention alone if the patient cannot accept or tolerate positive airway pressure or oral appliance therapy as a primary treatment. The focus of this chapter is to review these options.
Behavioral Interventions
Weight Loss
A detailed discussion of upper airway physiology in OSA is provided in Chapter 101. To place the importance of weight reduction as a target for intervention in many OSA patients it is important to recognize the pathophysiologic contribution of obesity to this disorder. This issue is discussed in depth in Chapters 101 and 115. The adverse effect of obesity on upper airway function may be mediated through several pathways, one of which is a direct influence on upper airway geometry. Studies in animals have indicated that upper airway resistance is influenced by mass loading of the anterior neck, which can simulate the clinical scenario of excessive adipose tissue deposits in this area.1 Further support for a significant pathophysiologic role of cervical obesity is provided by the observation that changes in pressure surrounding the neck are transmitted to the airway lumen and that cyclic pressure fluctuations in the pharyngeal fat pad coincide with intrapharyngeal pressure fluctuations.2,3 These data also support the importance of the observation that OSA patients have thick necks4 and that increased neck circumference is a predictive factor for OSA.5–7 Furthermore, velopharyngeal collapsibility increases with increasing neck circumference, at least in awake OSA patients.8
The presence of intrapharyngeal fat deposition may also be important in the pathophysiology of OSA (see Chapter 101). Several groups of investigators have observed increased intrapharyngeal adipose tissue or increased lateral fat pad size on computed tomography and magnetic resonance imaging of OSA patients.9–12 The significance of a space-occupying intrapharyngeal mass on pharyngeal function has been demonstrated in an animal model with increased upper airway resistance, which is related to the magnitude of inflation of a balloon catheter in the region of the upper airway lateral fat pad.3,13 In summary, upper airway closure during sleep partly depends airway size and shape as well as external tissue pressures from lateral pharyngeal fat pads exerting pressure on the airway lumen. Furthermore, to the extent that upper airway patency is increased by greater lung volume and reduced by decreased lung volume, greater truncal obesity leading to decreased lung volume may be another mechanism contributing to airway closure during sleep.
It has been well documented that either medical or surgical weight reduction can have a substantial positive impact on this disorder.14–21 In a prospective cohort study examining the association between weight change and the severity of sleep-disordered breathing, Peppard and colleagues demonstrated that a 10% weight loss predicted a 26% reduction in the apnea–hypopnea index (AHI) (Fig. 106-1). Smith and colleagues reported that a relatively modest weight loss provides significant benefit for some persons.22 Tuomilehto and colleagues reported the results of a randomized trial of a very low calorie diet with nutritional support compared to routine lifestyle counseling in patients with mild OSA; the total AHI was 9.3 ± 3.0 in the experimental group and 10.0 ± 3.0 in the control group. Weight loss was 10.7 kg in the experimental group and 2.4 kg in the control group. After 3 months of the weight loss intervention, the AHI in the experimental group was 5.3 and 8.1 in the control group. The 3-month AHI and lifestyle results were sustained over a 12-month period. The authors concluded that this lifestyle intervention was successful in treating mild OSA without resorting to positive airway pressure therapy.23
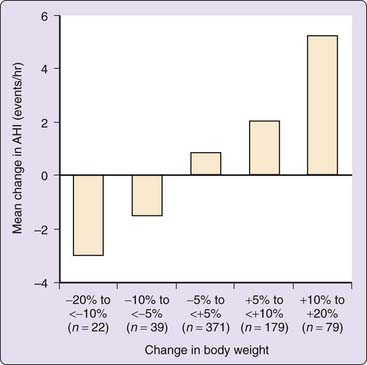
Figure 106-1 The effect of the change in body weight on the mean change in the apnea–hypopnea index (AHI) over a 4-year period.
(Redrawn from Peppard PE, Young T, Palta M, et al: Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000;284:3015-3021.)
Although it is clear that there remains a population of nonobese OSA patients and that some patients do not obtain benefit from weight reduction,16,18 obese patients should always be encouraged to lose weight. A multidisciplinary team approach to weight reduction, encompassing modification of lifestyle and diet as well as pharmacologic and surgical options, may optimize clinical results.24 Not only will this have a beneficial impact on overall health, it also has a high likelihood of reducing the severity of upper-airway dysfunction during sleep. The nonresponders might be those who have not lost sufficient weight, have coexisting craniofacial abnormalities, or have other operative pathophysiologic mechanisms such as perturbations of ventilatory control. Several important questions about weight loss and OSA remain unanswered, such as the effectiveness of weight loss in patients with more severe degrees of OSA, the effect of CPAP therapy on weight homeostasis, and the amount of weight loss that should occur before a reevaluation for OSA is recommended.
As discussed in detail in Chapter 115, bariatric surgery has gained popularity in the past decade as an alternative to diet and exercise for managing severe obesity. The preponderance of evidence to date suggests that this approach to weight loss brings short-term benefits for many obesity-related medical problems, including OSA.25,26 The long-term result of gastric bypass surgery on OSA is less clear, but results from one study demonstrated a 75% reduction in the respiratory disturbance index.26 Longer-term data are needed, but the short-term results of gastric-bypass surgery are encouraging.
Smoking Cessation
Tobacco use is known to have a detrimental effect on sleep. Cigarette users have more difficulty initiating and maintaining sleep and have increased daytime sleepiness.27 Although there are a variety of possible explanations for this, including the impact of nicotine withdrawal, it is conceivable that one mechanism is through an association between smoking and sleep-disordered breathing. Smokers have an odds ratio that is four to five times greater than that of never-smokers for having at least moderate sleep-disordered breathing.28 Cigarette smoking can contribute to upper airway dysfunction during sleep by eliciting mucosal edema and increased upper airway resistance. It is obvious that tobacco use should be discouraged on the basis of its adverse multisystem effects.
Early studies of nicotine-containing chewing gum suggested that it may be capable of reducing the AHI.29 However, subsequent studies have demonstrated that there was no clinically significant reduction in incidence of disordered-breathing events after application of transdermal nicotine patches, which have an extended duration of delivery. Apnea duration and snoring intensity was reduced, although the magnitude of the latter impact was insufficient to be therapeutically useful. Adverse effects were observed in conjunction with the transdermal patch including reduced total sleep time, sleep efficiency, and rapid eye movement (REM) sleep.30 Other side effects including gastrointestinal complaints, light-headedness, and tremor were also reported.
Sleep Hygiene and Sleep Deprivation
We live in a sleep-deprived society.31 In addition to the adverse impact of sleep-deprivation on performance, several lines of evidence suggest that absolute sleep deprivation, as well as repetitive sleep disruption, may also predispose to or worsen existing OSA. The effect of sleep deprivation on respiratory control mechanisms is unclear. Some studies have shown a blunted hypoxic and hypercapnic ventilatory chemoresponsiveness during wakefulness32,33 and may prolong apneas and hypopneas with consequently greater oxyhemoglobin desaturation, by depressing the arousal response,34,35 although one more-recent study has not.36 Another investigator has reported that upper airway collapsibility increased after sleep fragmentation but not following short-term sleep deprivation.37 The clinical importance of upper airway vulnerability induced by sleep restriction may be limited, but recommending patients obtain an adequate amount of sleep is a good practice for clinicians to adopt.
Body Position
A bed partner’s prompting to move to the lateral recumbent position is one of the oldest interventions for snoring and OSA. In many patients, the incidence of sleep-disordered breathing events is substantially greater during sleep in the supine position than in the lateral recumbent position.38–42 Sleeping in the supine position can increase the probability of upper airway occlusion due to the effect of gravity on the tongue, which tends to relapse posteriorly and come into apposition with the posterior pharyngeal wall. If the mass of soft tissue in the anterior cervical region enhances the propensity for upper airway dysfunction,1,4 it is reasonable to speculate that this factor interacts with body position to compromise upper airway patency during sleep.
Body position dependency is inversely related to the degree of obesity,38 with a greater likelihood that more obese patients have obstructive sleep apnea–hypopnea (OSAH) regardless of position. One should not assume that a patient with normal body weight has position-dependent OSAH.38 The presence or absence of obesity is not an invariable predictor of supine position–dependent sleep-disordered breathing. It has been suggested that there is no effect of body position specifically during REM sleep.42 Position-related alterations in overall incidence of apnea are present primarily during non-REM (NREM) sleep. There is no body position effect on apnea duration, suggesting that the arousal mechanism is influenced by sleep stage but not by body position.
Sleeping with the head and trunk elevated at a 30- to 60-degree angle to the horizontal has a favorable impact on OSA43; upper airway closing pressure (the magnitude of negative pressure when applied to the airway that results in occlusion) is more negative (indicating greater stability) in the 30-degree head-elevated position than in the supine and lateral recumbent positions during NREM sleep.44 Opening pressure (the magnitude of nasal continuous positive airway pressure [CPAP] that eliminates apnea, hypopneas, and oxyhemoglobin desaturation) is less in the head-elevated position than in the supine position but not significantly different from the lateral recumbent position. These data suggest that 30 degrees of head elevation may be more effective in stabilizing the upper airway than sleeping in the lateral recumbent position but that head elevation might not reduce the required magnitude of CPAP relative to lateral recumbency.
In addition to affecting the incidence of OSA, body position can influence central apnea. We have seen several patients with mixed and central apneas dependent on supine body position. Issa and coworkers45 also remarked on the presence of supine position–dependent central apnea in patients with predominantly obstructive apnea. It has been postulated that occlusion of the upper airway in the supine position is an important element in the pathogenesis of these central apneas. Sanders and colleagues46 suggested that upper airway obstruction during exhalation could result in prolonged, low-level expiratory airflow, which is generally below the threshold for detection by usual recording techniques, thereby simulating true central apnea. Several additional reports, employing video endoscopy, have demonstrated the presence of upper airway occlusion during otherwise central apneas.47,48 Issa’s group45 postulated that supine position–dependent (gravity-related) apposition of the pharyngeal walls precipitates central apnea via reflex inhibition of ventilation. The tendency for improvement of these central apneas by nasal CPAP45,46,49 or, in some cases, by maintaining the lateral recumbent body position supports a pathophysiologic role of upper airway obstruction in these events.
Alcohol
Bed partners and housemates are probably the first to recognize the association between alcohol consumption and upper airway dysfunction during sleep. A discussion of the physiologic effect of alcohol on the upper airway is provided in Chapter 132. It is clear that alcohol evokes obstructive apnea in persons who otherwise only snore and increases the apnea frequency in patients with preexisting OSA.34,50–53 There are limited and conflicting data regarding the contribution of alcohol to the development of upper airway dysfunction in persons who otherwise do not snore or have sleep apnea.51,52 In addition to increasing the frequency of sleep-disordered breathing events, alcohol consumption increases the duration of these events.54,55
Alcohol may also have a particularly adverse impact on daytime alertness in OSAH patients. The hypnotic effect of this agent is enhanced in the presence of underlying sleepiness. Alcohol consumption superimposed on sleepiness, as in sleep deprivation, is associated with worse performance on driving simulator tests compared with sleep deprivation and placebo.56 It would be expected that alcohol would have a greater detrimental impact on daytime alertness in OSA patients who have increased basal sleepiness.
There is inconsistency in the literature regarding the impact of alcohol on the magnitude of the positive pressure prescription,57–59 and patients who are unlikely to alter their alcohol consumption should have a therapeutic trial under usual lifestyle conditions. Therapy needs to be proved successful despite the presence of exogenous as well as endogenous influences on the pathophysiologic processes. This includes alcohol consumption as well as other factors, including body position during sleep (some persons may only be able to sleep in the supine position) or unavoidable need for treatment with pharmacologic agents that can adversely effect upper airway stability during sleep (e.g., benzodiazepines).
Sedatives and Hypnotics
An overview of the physiologic impact of sedatives and hypnotics on sleep and breathing is provided in Chapter 110. Although the effect of every individual benzodiazepine on breathing during sleep has not been evaluated, flurazepam has been the subject of several investigations. This agent can worsen OSA in some persons who otherwise have mild OSA.60–62 Reports addressing the impact on breathing during sleep in otherwise healthy subjects provide inconsistent results.62,63
A limited number of studies have examined the impact of benzodiazepines other than flurazepam and diazepam. In a group of patients with mild insomnia and sleep apnea (AHI, 8.8 ± 5.3, mean ± SE), 15 to 30 mg of temazepam did not significantly increase the AHI or alter the degree of oxyhemoglobin desaturation, compared with placebo.64 In a group of patients with mild chronic obstructive pulmonary disease, no significant increase in the group average AHI was observed following 0.25 mg of triazolam.65
Due to the small populations evaluated in published investigations as well as the conflicting results of these studies, the safety of benzodiazepine administration to OSA patients remains uncertain and the issue remains debated.66 Although in usual hypnotic doses, benzodiazepines might not present a substantial risk for evoking OSA in some otherwise normal persons, in view of the inconclusive data regarding the margin of safety, it is prudent to avoid this class of agents in patients who have OSA and those with risk factors for this disorder.
Narcotics and Anesthetics
There have been anecdotal reports of clinically significant upper airway obstruction developing after administration of intravenous narcotics.67–69 This is discussed in Chapter 132. Normal subjects failed to demonstrate a change in breathing during sleep after oral administration of hydromorphone hydrochloride in 2-mg and 4-mg doses.70 It might not be valid to extrapolate these results to the administration of higher doses of narcotics or give them to patients who have or are at risk for upper airway dysfunction during sleep. It is prudent to withhold, or at least minimize, narcotics given to patients with OSA or those with risk factors for this disorder, depending on the clinical circumstances.
In instances when humane medical practice requires analgesia, such as in the postoperative or periprocedure setting, if nonnarcotic or nonventilatory depressant agents cannot provide adequate pain control, and if the risk is deemed clinically acceptable, minimal doses of narcotics should be administered with careful monitoring. Patients who are already using CPAP for OSA should use this therapy in the postoperative and periprocedure periods. They may have increased pressure requirements in the presence of narcotics and following general anesthesia. General anesthesia can also promote upper airway instability by selectively reducing the muscle tone to the upper airway dilator muscles.71 If patients have not previously required CPAP therapy for OSA, empirical use of CPAP can be considered until there is no longer a need for drugs with the potential to affect ventilation or upper airway function.
Attention has been paid to the influence of higher-potency narcotics on ventilation during sleep. Several reports have linked opioids to central sleep apnea. On the basis of two relatively small studies, the prevalence of methadone-induced central sleep apnea is about 30%.72,73 Some studies have shown that obstructive sleep apnea is more common in this group.73a Clinicians should be aware of the potential for higher-potency opioids to cause central or obstructive sleep apnea and to consider this in the differential diagnosis of sleep-disordered breathing when evaluating such patients.
Barbiturates
There have been no systematic assessments of the impact of barbiturates on upper airway function during sleep. Like alcohol, this class of agents selectively reduces the neural output via the hypoglossal nerve, reduces the tone of the upper airway dilator muscles, and predisposes to upper airway occlusion during sleep.74,75 It is prudent to avoid barbiturates in patients who are predisposed to or are known to have sleep-disordered breathing.
Other Agents
Phosphodiesterase-5 Inhibitors
It is not uncommon for erectile dysfunction and OSA to coexist in patients.76 Phosphodiesterase 5 inhibitors such as sildenafil are being used to treat erectile dysfunction and pulmonary hypertension (which also can coexist with OSA). This class of agents inhibits degradation of cyclic guanosine monophosphate (cGMP) and as a consequence they increase nitric oxide (NO) expression. Nitric oxide is associated with smooth muscle relaxation and vasodilation. It can promote nasal congestion and adversely influence hypoxic vasoconstriction in the pulmonary vascular bed and in doing so can increase the propensity for obstructive apneas and hypopneas as well as amplifying the degree of oxyhemoglobin desaturation in conjunction with these events. Along these lines, a double-blind, randomized, controlled study in 14 patients with moderate to severe OSA (AHI, 31.6; 95% confidence interval [CI], 23.6 to 39.5) found that compared with values after placebo, 50 mg of sildenafil before bedtime was associated with increased percentage of the total sleep time during which oxyhemoglobin saturation was less than 90%, a decrease in the mean SaO2, and increased AHI.77 The authors concluded that sleep-disordered breathing should be considered in all patients with erectile dysfunction, and when erectile dysfunction is present, caution should be used when considering prescription of sildenafil.
Testosterone
Although controversial, there is a body of mostly anecdotal literature suggesting that treatment with testosterone can worsen upper airway function and exacerbate OSA. This topic is discussed in detail in chapter 125. Although more definitive information regarding the relationship between testosterone replacement therapy and OSA is needed, it would seem prudent for clinicians to explore the possibility of OSA in patients before prescribing testosterone and counsel patients who are to receive it about symptoms and signs. A high clinical suspicion for OSA should be maintained in patients receiving this therapy.
Endocrine Considerations
Hypothyroidism
The physiologic relationship between thyroid function and sleep-disordered breathing is addressed in Chapter 125. Although the magnitude of the clinical association between hypothyroidism and OSA is not clear, there is considerable researech in support of an association between the two conditions.78–81
Thyroid hormone replacement therapy might benefit OSAH patients by reducing the incidence of apnea.80 On the basis of the data suggesting that thyroid hormone replacement improves upper airway function during sleep, clinicians should consider the possibility of hypothyroidism adversely affecting OSAH. Hypothyroid patients (and their bed partners) should be specifically interviewed and examined to detect factors that suggest OSAH is present.
Although OSAH may be reduced after initiating thyroid hormone replacement therapy, the degree of improvement may be insufficient to obviate the need for additional treatment.80,82 It is important to repeat an objective assessment of breathing during sleep after restoration of the euthyroid state. Grunstein and colleagues82 made the important observation that thyroid hormone replacement in patients with untreated OSA can precipitate cardiac complications attributable to ischemia in the setting of augmented metabolism and persistent nocturnal hypoxemia. For this reason, as well as the benefit of more-rapid relief of OSA, the authors suggested treatment with nasal CPAP during thyroid replacement. This recommendation could be extrapolated to include successful oral appliance therapy. This strategy appears prudent until objective reassessment is performed when the patient is clinically and chemically euthyroid and further management decisions can be made based on the subsequent reassessment.
The utility of screening unselected OSAH patients for hypothyroidism is controversial. Winkelman and coworkers have reported a similar prevalence of chemical hypothyroidism in OSA patients and in persons without OSA.83 These authors concluded that routine screening for thyroid dysfunction is not indicated in OSAH patients unless there are corroborating signs and symptoms. The possibility of concomitant hypothyroidism should be considered in patients who have OSA and are receiving adequate therapy but who continue to report symptoms of fatigue and daytime sleepiness.
Estrogen and Progesterone
The effect of gender on OSAH has been well defined; the male-to-female ratio is 2 or 3 : 1.84 After menopause, the risk of OSA increases.85,86 The exact mechanism(s) conferring this risk are incompletely understood, but they appear to be in part a function of estrogen and progesterone levels.87 Older reports have demonstrated that hormone replacement therapy (HRT) has a favorable effect on the OSA.85,86 However, the additional risks as defined by the Women’s Health Initiative study likely outweigh the potential benefit. Further research might define subsets of patients and dosing regimens that are safe and effective. It is unlikely that this intervention would be robust enough to preclude the use of positive pressure in severe OSA.87
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


