17
Microvascular Decompression for Hemifacial Spasm and Trigeminal Neuralgia
The microvascular decompression of cranial nerves has become an accepted surgical technique for the treatment of trigeminal neuralgia, hemifacial spasm (HFS), glossopharyngeal neuralgia, and other cranial nerve neuropathies. It is a surgery that requires sound anatomic knowledge and surgical finesse. The cerebellopontine angle has important vessels and nerves that may be damaged, leaving the patient with severe deficits. The patients are generally healthy except for the neuralgia; therefore, such deficits are unacceptable.
This chapter is based on the surgical experience of over 837 cases since 1980, and we discuss the lessons we have learned from our complications and failures.
♦ History
Observations of vascular compression of the seventh cranial nerve in patients with HFS were first reported by Campbell and Keedy1 in 1947 and by Laine and Nayrac2 in 1948. Gardner and Sava3 in 1962 proposed decompression of the facial nerve for HFS. Nicolas André coined the term tic douloureux, and in 1756 discussed surgical treatments in five patients who suffered with a “cruel and obscure illness, which causes . . . in the face some violent motions, some hideous grimaces, which are an insurmountable obstacle to the reception of food, which put off sleep.”4 In 1966, Jannetta and Rand5 proposed that the etiology of trigeminal neuralgia (TN) lies in the compression of the trigeminal nerve by small vessels near the brainstem. Since that time, microvascular decompression (MVD) has become a mainstay in the management of this painful condition.
♦ Procedure
The operative steps are described and illustrated in the following subsections. The steps are designed to achieve clinical pain relief while avoiding potential complications.
Intraoperative Monitoring
Facial nerve monitoring is done in cases of HFS, but we have found that the results of monitoring do not always correlate with clinical results.6 Intraoperative monitoring of brainstem auditory evoked potentials (BAEPs) is a useful tool to decrease the danger of hearing loss, and we use it in most cases.
Positioning of the Patient
The success of the microvascular decompression procedure is significantly determined by patient positioning. The authors prefer the modified park-bench position (Fig. 17.1). Following anesthesia and intubation, a Sugita head-fixation device is applied and the patient is placed in the lateral decubitus position with appropriate padding of pressure points and an axillary roll. The neck is flexed slightly with the chin approximately two fingerbreadths from the sternum. The head is rotated approximately 10 degrees away from the affected side. If a trigeminal or cochlear approach is planned, the vertex is kept parallel to the floor to keep the cranial nerve VII-VIII complex at a more inferior position in relation to the trigeminal nerve. The head holder is secured in the proper position. The patient is fixed securely with belts to the table at the hip and chest to allow rotation of the table during the operation. The patient’s shoulder is taped down and placed out of the way.
Surgical Incision
A limited area behind the ear is shaved, and bony landmarks are identified by palpation before the patient is prepared for surgery. The surgeon should have an idea of the position of the transverse and sigmoid sinus for proper bur-hole placement. An S-shaped incision beginning approximately 5 cm above the midmastoid is taken down 3 cm posterior to the inferior edge of the mastoid tip (Fig. 17.2). Three quarters of the incision is drawn inferior to the junction of the transverse and sigmoid sinuses and one quarter above. This curved incision lowers the height of reflected skin and subcutaneous tissue on the surgeon’s side (Fig. 17.3).
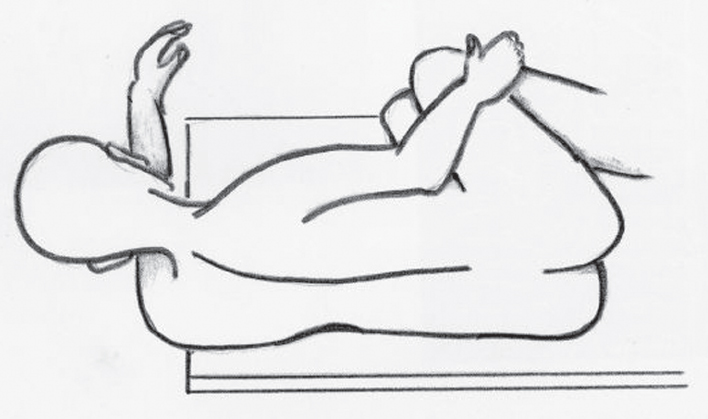
Fig. 17.1 The modified park bench position.
The incision is extended down to the occipital bone by using monopolar cautery. The soft tissues are dissected using a periosteal elevator and electrocautery where necessary. The nuchal muscles are dissected with electrocautery and the transverse occipital artery is ligated and divided. The mastoid emissary vein usually bleeds and is waxed in its fossa. An angled mastoid retractor is put in place. The loose fatty tissue at the inferior aspect of the digastric groove is preserved to avoid injury to the vertebral artery and condylar vein.

Fig. 17.2 The S-shaped incision.
Craniotomy
Before bur-hole placement, bone landmarks should be well exposed. The bony exposure should be enough to identify the edge of the junction of the transverse and sigmoid sinuses. A small craniectomy can then be tailored according to the cranial nerve approach desired. The craniotomy for HFS is shaped like an inverted triangle with a 3-cm maximum dimension. The craniotomy for trigeminal neuralgia is more elliptical and also more cranial by approximately 1 cm (Fig. 17.4). The advantage of the inverted triangle is that the retractor can be moved along the sigmoid sinus to visualize from the cranial nerve VII-VIII complex to the IX-X-XI complex. Mastoid air cells should be thoroughly waxed. The bone work must be anterior enough to allow dural incision and reflection (Fig. 17.5).
Dural Opening
The surgeon should allow some drainage of cerebrospinal fluid (CSF) before placing the microretractor. As the CSF is being drained, the cerebellum will begin to fall away from the cerebellopontine angle. Some unpleasant bleeding from the torn bridging veins may be encountered, which can be managed by suction over a cottonoid and bipolar diathermy. We usually try to identify the VII-VIII complex through the arachnoid. In cases of HFS, we cut the arachnoid down to the lower cranial nerves and in TN we cut it up to cranial nerve V and the petrous vein. It is important to open the arachnoid with sharp dissection to see the root entry zone (REZ). In HFS, the arachnoid is cut over the VII-VIII complex, the IX-XI complex, and choroid plexus (Fig. 17.6).
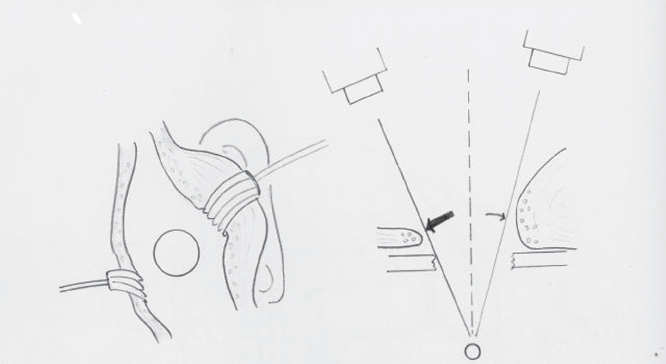
Fig. 17.3 The advantage of the curved incision is that it lowers the height of reflected skin and subcutaneous tissue on the surgeon’s side.
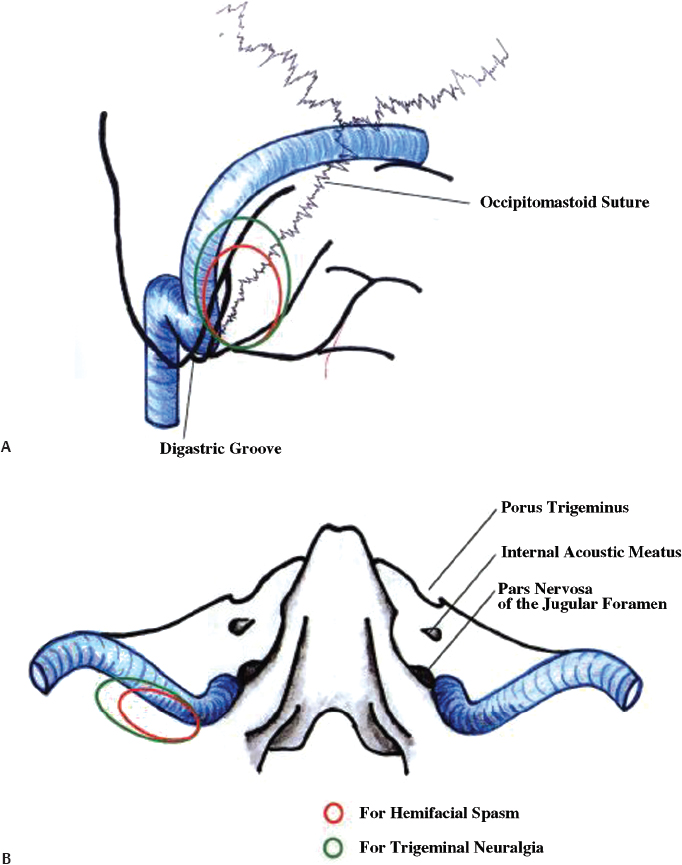
Fig. 17.4 The schematic relation of the craniotomy to the bony landmarks and venous sinuses. (A) posterior view; (B) inferior view.
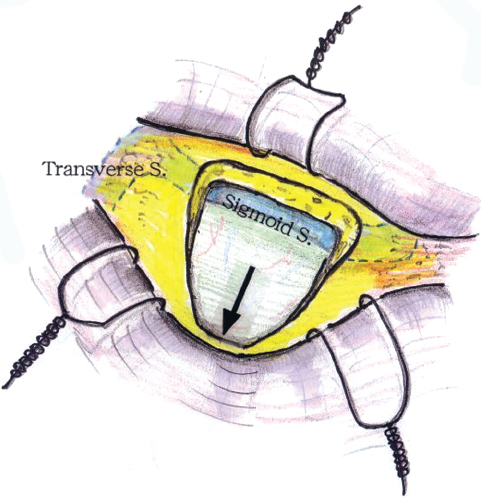
Fig. 17.5 The inverted triangle craniotomy for hemifacial spasm.
The senior author does not hesitate to use retractors until the REZ becomes visible. It is also easy to elevate the arachnoid membrane with suction. The large size of the flocculus in some patients makes it necessary to use a retractor to see the REZ (Fig. 17.7). After this, retractors can be avoided altogether to prevent pull on cranial nerves VII and VIII. In cases of TN we prefer the lateral approach of identifying the VII-VIII complex and then moving cranially to cranial nerve V. It is better to apply retractors after the petrosal vein is lax.

Fig. 17.6 The extent of arachnoid dissection in microvascular decompression. Mem., membrane. 1, first the arachnoid over the facial nerve is dissected, and then the dissection continues (2).

Fig. 17.7 A large flocculus makes it necessary to use a retractor to see the root entry zone (REZ). (A) pre-retraction; (B) post-retraction (the arrows show the direction of the retraction).
Identification of Offending Vessel and Nerve Decompression
The basis of surgery in HFS is the vessel compression at the REZ where the oligodendroglia changes to the myelin sheath (Fig. 17.8). The common vessels causing compression of the facial nerve are the posterior inferior cerebellar artery (PICA) and the anterior inferior cerebellar artery (AICA). The vessel must be sharply dissected free from the arachnoid and mobilized laterally away from the nerve so that a Teflon implant can be placed (Fig. 17.9). Fibrin glue is applied over the Teflon to prevent slippage. In cases of atypical HFS, the pathologic vascular entity is almost always located rostral to the nerve or between cranial nerves VII and VIII. When the anterior inferior cerebellar artery is transposed laterally, a small branch to the pons may get stretched.
Compression of cranial nerve VII by the vertebral artery is a more challenging job for the surgeon. The artery may compress the nerve all along the course or at the REZ behind the cranial nerve IX (Fig. 17.10). The AICA also may run along the nerve, but decompression of only the AICA does not relieve the facial spasm. Vertebral artery compression has to be treated by transposition and not Teflon alone. The vertebral artery is transposed laterally, wrapped in Surgicel and Teflon, and coated with fibrin glue or cyanoacrylate (Fig. 17.11). Microvascular decompression of the trigeminal nerve requires sharp dissection of all arachnoid around the trigeminal nerve and superior cerebellar artery. The most common vessel found is a superior cerebellar artery loop, which compresses the trigeminal nerve either at the brainstem or distally (Fig. 17.12). The nerve should be inspected from its origin at the brainstem laterally to its exit from the cerebellopontine angle, and all vessels should be treated. After the arachnoid is dissected and the vessel is freed, the loop can be mobilized to the lateral aspect of the nerve, and a piece of shredded Teflon felt can be placed between the vessel and the nerve.

Fig. 17.8 The transition zone from the oligodendroglia to the myelin sheath is compressed by the vessel.
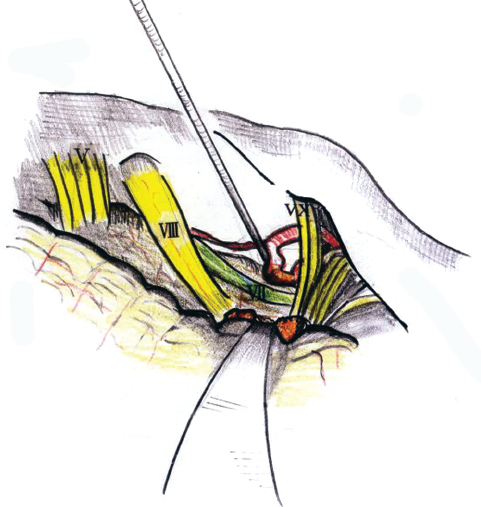
Fig. 17.9 This figure shows the standard vascular compression.
In some cases, no offending artery is found but a vein is identified running over the REZ of cranial nerve V. This vein must be ablated. Sometimes a branch of the petrosal vein may be the offending vessel and needs to be ablated (Fig. 17.13). It is important not to ablate all the petrosal vein branches. Brain retraction must be avoided after petrosal vein ablation to prevent hemorrhagic venous infarction. In certain cases, despite performing a thorough search, no compressing vessel is found but cranial nerve V looks kinked. This kinking itself can produce neuralgia and needs to be corrected.
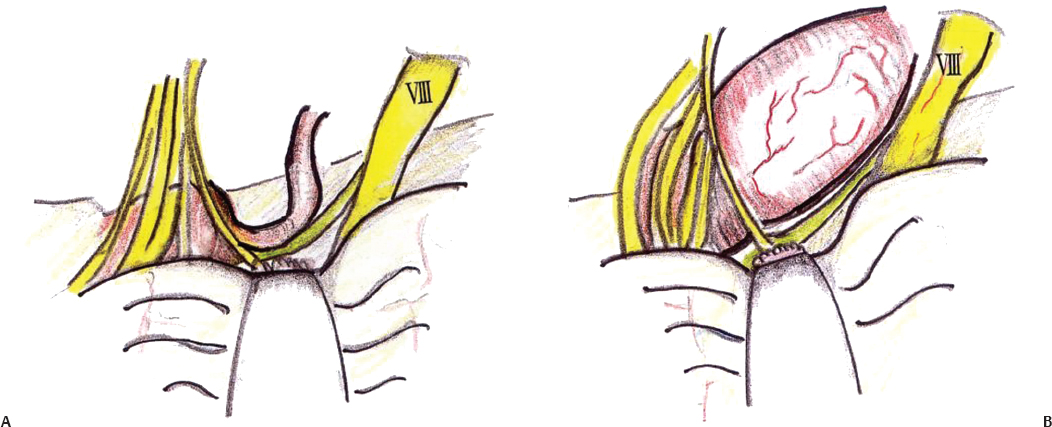
Fig. 17.10 (A) Partial compression on cranial nerve VII by the vertebral artery. (B) Complete compression on cranial nerve VII by the vertebral artery.
Closure
At the conclusion of the procedure, repetitive Valsalva maneuvers are performed to ensure hemostasis. The retractor is then removed and the cerebellar surface is carefully inspected. The area is gently irrigated with warm saline and the dura is closed. We insist on watertight dural closure in which fascia/muscle grafts from the inferior portion of the incision are used when necessary. The bone edges of the mastoid air cells are thoroughly waxed for a second time. A small strip of polyglycolic acid is placed over the suture line and fibrin glue is applied over it. Gelfoam is then placed over the durotomy. The bone defect may be either repaired by titanium mesh or left alone. The deep and superficial muscles are approximated with interrupted No. 2-0 absorbable sutures, and the fascia is closed with the same type of sutures. The fascial closure has to be good to prevent any CSF leakage. We prefer interrupted sutures. The subcutaneous tissues are approximated with No. 3-0 interrupted absorbable sutures. A well-approximated subcutaneous layer can also afford protection from CSF leakage. The skin is closed with No. 4-0 nylon or staples.
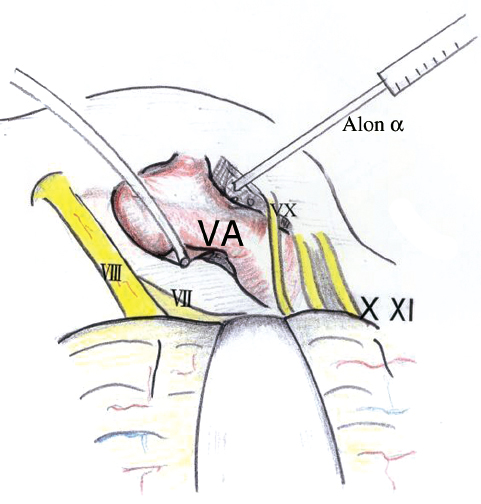
Fig. 17.11 Transposition of the vertebral artery (VA). Alon α, fibrin glue.

Fig. 17.12 Compression of cranial nerve V by the superior cerebellar artery (Sup. Cerebellar A.).
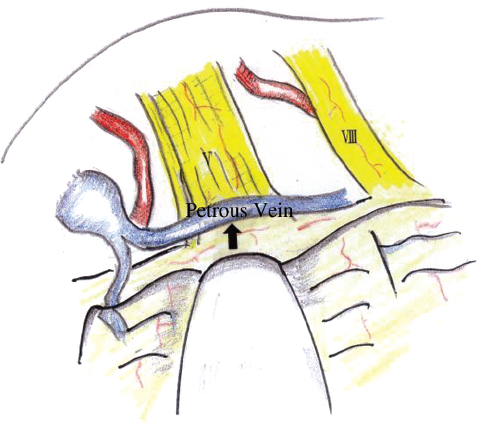
Fig. 17.13 Venous compression on cranial nerve V.
Use of Endoscope in Microvascular Decompression
Why do some microvascular decompression procedures fail? One reason is misidentification of the compressing vessels. The aim of the endoscope is to increase the yield of compressing vessels. With the endoscope, we can visualize areas behind the nerves, vessels, flocculus, and cerebellum that are not visible to the microscope. We use the Machida right-angled endoscope, which is easy to handle and safe (Fig. 17.14). In two or three cases out of 50 it provides the neurosurgeon with additional information. The space behind cranial nerve V can be deep and large veins traverse the space. Endoscopy is useful to look at the details of this space. It is of particular importance when the nerve is compressed from behind, so that the compression point can still be seen clearly and tackled (Fig. 17.15).
Failure of Microvascular Decompression
Even for experienced surgeons, it is still impossible to accomplish a 100% cure rate. Most series report 85 to 96% cure rates after microvascular decompression surgery. Recurrence is defined as persistent or diminished facial spasm after a period of complete resolution after microvascular decompression.7 The authors have performed 837 procedures of microvascular decompression, of which 550 were for HFS and 287 for trigeminal neuralgia. We assess the cure rates for microvascular decompression in cases of HFS 12 months after surgery. We had a cure rate of 95.3% up through 2001, and have analyzed the cases of recurrence that we managed and did a literature review to postulate certain causes of recurrence.
Fig. 17.14 A Machida endoscope.
Wrong Insertion of Teflon Patch
It is important to insert the Teflon patch at the REZ of cranial nerve VII, which is located behind cranial nerve IX. Peripheral insertion may lead to recurrence. At the second surgery, lower cranial nerves were carefully dissected and the REZ was well exposed. Decompressions were made at the level of REZ with good results.
Missed Compressive Element
Sometimes the vascular compressive element may be missed on initial surgery. The initial pain relief may be because of partial nerve injury. We have had two cases in which the internal auditory artery was found as the compressive element at reexploration (Fig. 17.16).
Sometimes both the AICA and the vertebral artery may be compressing the nerve (Fig. 17.17). One of them may be missed at the initial surgery. Here once again, we insist on the need to look at the nerve from the REZ to the periphery. Venous compression is generally undertreated and may lead to recurrence.
Slippage or Decomposition of the Teflon Sponge
Many times at reexploration, the muscle plug used during the previous surgery was found to have decomposed. This is rarely seen with Teflon. To prevent slippage we use Surgicel and glue on the Teflon patch.
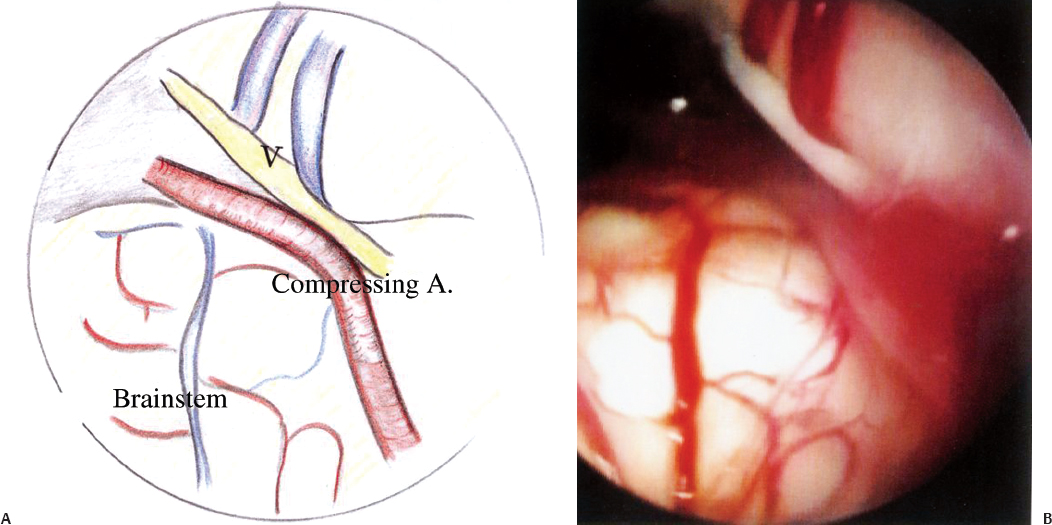
Fig. 17.15 Diagrammatic (A) and endoscopic (B) views of the vessel compressing cranial nerve V.
Scarring
The implant itself may produce severe scarring in some cases, causing nerve deficits and recurrence.8 Hence, it is important to use thin-shredded Teflon in optimum quantities. We have had a case in which a large patch was demonstrated on postoperative computed tomography (CT) scan causing facial palsy. The patient was reexplored and the patch was removed.
Demyelination
Bederson and Wilson9 reported in 1989 that prolonged extrinsic compression may lead to intrinsic demyelinating lesion, which will not respond to decompression. Reexploration of such patients may not lead to any compressive element, and a partial rhizotomy or other ablative procedures may have to be considered.

Fig. 17.16 (A) At first surgery, only the anterior inferior cerebellar artery (AICA) was transposed. (B) At the second surgery, the internal auditory artery was also found compressing the nerve and was decompressed. The arrows indicate the insertion of the Telfa (cottonoid) in the plane between the artery and the nerve.

Fig. 17.17 Wrong insertion of a patch between cranial nerve VII and the AICA without pain relief.
♦ Complications
The complications encountered in our series included cerebellar injury and CSF leakage. Other complications of the procedure such as facial weakness or anesthesia, hearing loss, and lower cranial nerve dysfunction were rare. Most cases with postoperative facial weakness improved within 3 months. Unfortunately, one patient required plastic surgery for the facial palsy. Another patient lost hearing completely.
The following surgical errors may lead to facial paresis or palsy:
• Direct nerve injury
• Extensive coagulation near the facial nerve
• Wrong tangent of retraction: retraction should never pull on the cranial nerve VII-VIII complex and should be avoided after the REZ is exposed
• Large Teflon patch, as already mentioned, may itself produce neuralgia
• Postoperative viral infection, commonly varicella zoster virus
♦ Conclusion
Microvascular decompression is one of the most gratifying procedures in neurosurgery. Proper surgical techniques can alleviate the patient’s symptoms and make it an excellent operation for a painful disease.
References
1. Campbell E, Keedy C. Hemifacial spasm; a note on the etiology in two cases. J Neurosurg 1947;4:342–347 PubMed
2. Laine E, Nayrac P. Hemispasme facial guéri par intervention sur la fossa postérieure. Rev Neurol 1948;80:38–40
3. Gardner WJ, Sava GA. Hemifacial spasm—a reversible pathophysiologic state. J Neurosurg 1962;19:240–247
4. Brown JA, Coursaget C, Preul MC, Sangvai D. Mercury water and cauterizing stones: Nicolas André and tic douloureux. J Neurosurg 1999;90: 977–981 PubMed
5. Jannetta PJ, Rand RW. Transtentorial retrogasserian rhizotomy in trigeminal neuralgia by microneurosurgical technique. Bull Los Angeles Neurol Soc 1966;31:93–99 PubMed
6. Kanno T. Microvascular Decompression for Hemifacial Spasm and Trigeminal Neuralgia. 2nd ed. Japan: Neuron Publishing, 2006.
7. Payner TD, Tew JM Jr. Recurrence of hemifacial spasm after microvascular decompression. Neurosurgery 1996;38:686–690, discussion 690–691 PubMed
8. Kureshi SA, Wilkins RH. Posterior fossa reexploration for persistent or recurrent trigeminal neuralgia or hemifacial spasm: surgical findings and therapeutic implications. Neurosurgery 1998;43:1111–1117 PubMed
9. Bederson JB, Wilson CB. Evaluation of microvascular decompression and partial sensory rhizotomy in 252 cases of trigeminal neuralgia. J Neurosurg 1989;71:359–367 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








