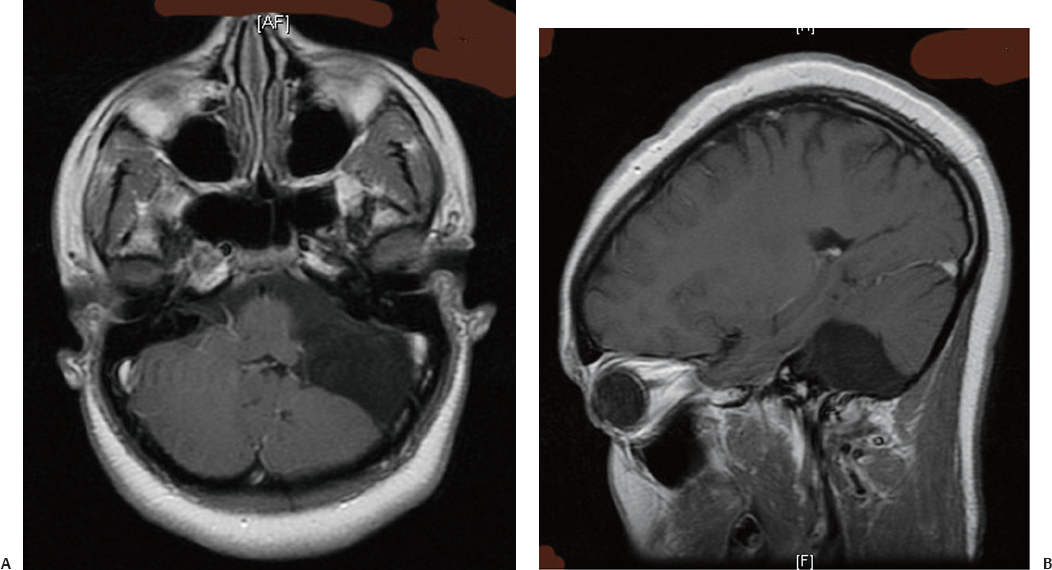
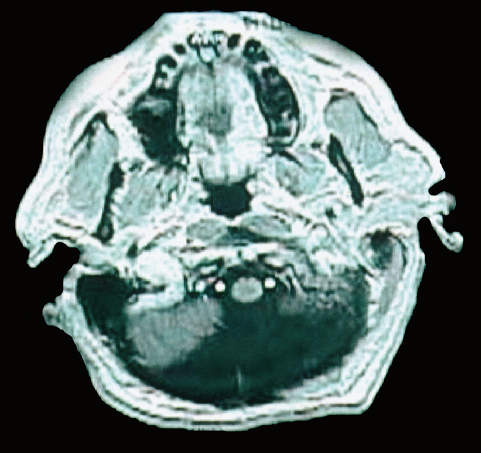
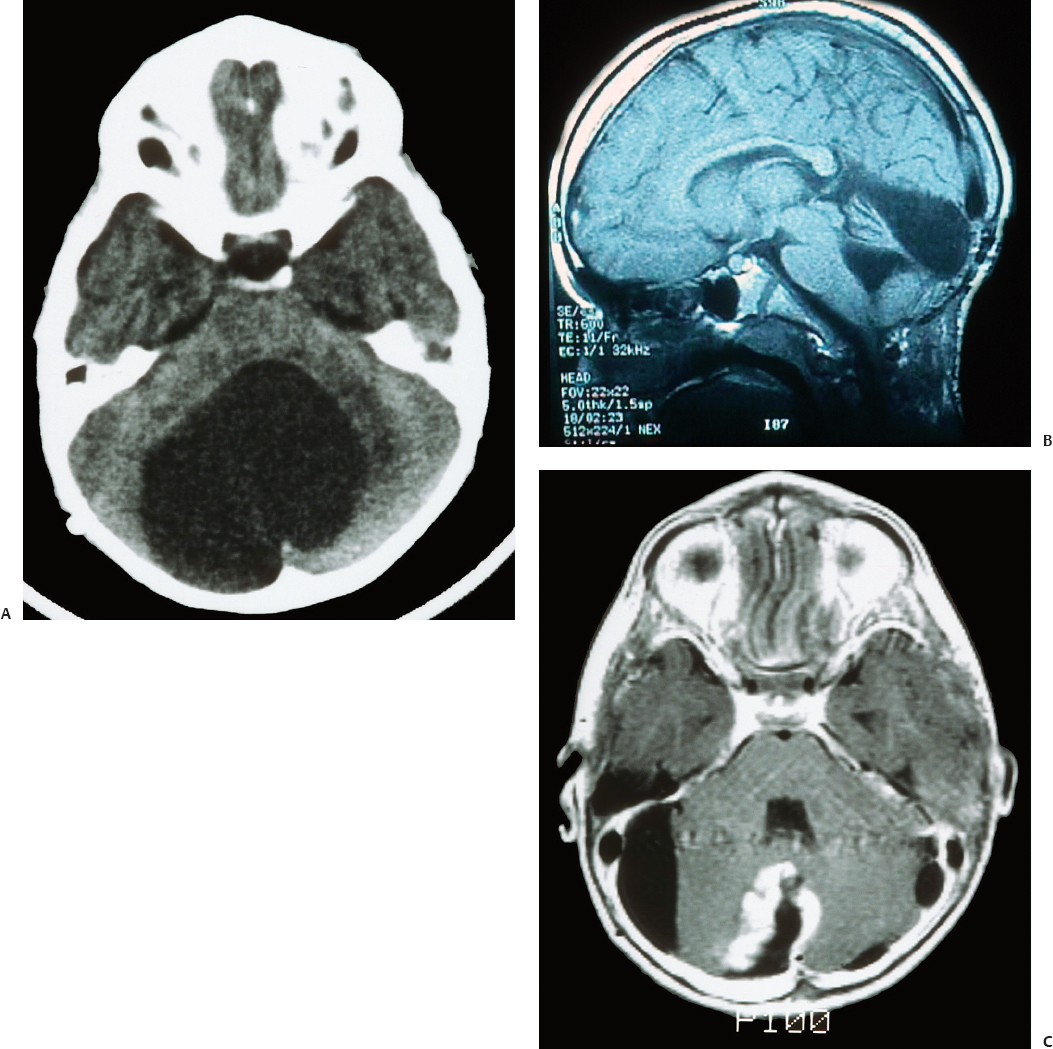
24 Numerous forms of treatment have been suggested and tried, but, as the number of methods indicates, they have been almost uniformly unsuccessful. The etiology being so obscure, any treatment is necessarily empirical and consequently unsatisfactory. Successful therapy must depend on the identification and the treatment of cause of the disease.1 The above statement appears in “Internal Hydrocephalus: An Experimental, Clinical, and Pathological Study,” by Dandy and Blackfan, who studied hydrocephalus extensively in both clinical and experimental settings. Historically, the existence of a foramen (foramen of Magendie), leading from the fourth ventricle to the subarachnoid space, was suggested by Haller and Cotugno. In 1764, Cotugno2 first proved the existence of the subarachnoid space and also found fluid in this space in aquatic animals. Magendie made an important contribution to the understanding of cerebrospinal fluid (CSF) pathways by demonstrating in animals that fluid normally fills the ventricles and the subarachnoid space. He showed that a free communication exists between the ventricles and the subarachnoid space by means of the foramen, which was named after him. The central and spinal subarachnoid space forms a single freely communicating space. Magendie observed that the aqueduct of Sylvius or the foramen of Magendie was obstructed in several cases of hydrocephalus. The important issue of formation and absorption of CSF has been eloquently described by Key and Retzius3 and Dandy and Blackfan.1 Even today, the majority of their experimental results remain mostly valid. Interestingly, the treatment proposed prior to the introduction of shunts for hydrocephalus in the early 1950s was to tackle the primary disease effectively. Present-day trends seem to consider this philosophy as well. Several studies have been performed to establish clear indications for ventricular shunts, and many are still trying to do so. In 1881, Wernicke performed the first ventricular puncture and external CSF drainage. Quincke introduced lumbar puncture a decade later. Mikulicz reported the first ventriculo-subarachnoid-subgaleal shunt in 1893.4 A lumboureteral shunt was used successfully based on the concept of Heile, who sutured the renal pelvis to the dura and arachnoid.5 In 1918, Dandy6 introduced choroid plexectomy, and Torkildsen7 described ventriculocisternostomy. Based on the pioneering work of John Holter and Eugene Spitz, valve regulated shunt systems were introduced, and the first such shunt surgery was reported in 1949 by Nulsen and Spitz. Several modifications and technological advances resulted in more than 130 different designs.4 Posterior fossa lesions contribute to the development of the majority of hydrocephalus seen in younger patients. The lesions could be congenital, infective/inflammatory, or neoplastic. This chapter discusses the role of shunts in these different pathologies affecting posterior fossa structures (Fig. 24.1). In 1914, Walter Dandy and his pediatrician colleague Kenneth Blackfan described a group of congenital anomalies associated with cerebellar agenesis and a cystic lesion of the posterior fossa. Although Sutton8 described such an entity earlier, the details of this syndrome, demonstrated succinctly in autopsy specimens by Dandy and Blackfan, and later by Taggart and Walker,9 came to be known as the Dandy-Walker syndrome (DWS). The term was coined by Benda,10 who also pointed out that DWS is primarily a developmental anomaly of the fourth ventricle with membrane alterations and a cerebellar cleft. Benda also ruled out atresia of the foramina of Luschka and Magendie as originally put forth by Dandy. Gardner11 emphasized the disparity between the rate of formation and rate of egress of cerebrospinal fluid (CSF) from the neural tube at an early stage prior to the opening of the foramina of the fourth ventricle. DWS is characterized by cystic dilatation of the fourth ventricle, hypoplasia of the cerebellar vermis, and hydrocephalus. The presence of a posterior fossa cyst with varying degrees of dysgenesis is essential for the diagnosis of this disorder (Fig. 24.2). Fig. 24.1 Axial (A) and sagittal (B) magnetic resonance imaging (MRI) scans of an arachnoid cyst, which is a more common entity in the posterior fossa, showing the compressed fourth ventricle and cerebellar vermis. The cerebellar hemispheres are also seen to be normal but compressed on the ipsilateral side. For treatment purposes, DWS can be similar to retrocerebellar arachnoid cyst (Blake’s pouch cyst) and other variants, although the developmental origin differs. It is also noteworthy that hydrocephalus may not be an accompanying component of all cases of DWS.12,13 Dandy14 and Sahs15 advocated direct fenestration and communication of the cyst with the subarachnoid space, but with high mortality. The vast majority of these patients in later reports ended up with the placement of shunts.16,17 The primary mode of treatment is shunting. Various shunt procedures, such as ventriculoperitoneal (VP) shunt, cystoperitoneal (CP) shunt, and a combination of VP and CP shunts, are considered in the management options. Carmel et al18 suggested that a free communication exists between the cyst and ventricular system and proposed shunt placement in the lateral ventricle. They also proposed that the cyst-ventricle relationship may not remain constant throughout life, and that the free communication may be lost in the natural history of the disease as well as in the ventricular shunting procedure. For this reason, Raimondi et al19 advocated a combined ventricular and cyst shunt procedure (VP and CP shunts). Based on extensive manometric, radiologic, and pathologic examinations, a Y connecting system was also recommended for equalization of pressures inside the ventricles and the cyst. This procedure found support from many others.20,21 In case of patency of cystoventricular communication, a cystoperitoneal shunt alone is suggested to be adequate because it drains both the cyst and the ventricles.22 The experience from the Hospital for Sick Children, Toronto, Canada,12 demonstrated that stenosis of the aqueduct comes as an acquired sequela because the interval between an initial VP shunt and the next CP shunt varied between 6 months and 15 years. Two separate earlier reports by Foltz and Shurtleff23 and Hawkins et al24 also pointed out that inserting a shunt into the lateral ventricle can lead to the development of an acquired aqueduct stenosis. Domingo and Peter13 suggested from their experience with 50 cases of posterior fossa cysts that CP shunts encourage normal flow of CSF through the aqueduct and consequently reduce the incidence of aqueduct stenosis. Fig. 24.2 Axial section of posterior fossa computed tomography (CT) scan showing a typical Dandy-Walker cyst. There is agenesis of the vermis, and major portions of the cerebellar hemisphere and the cyst may be in communication with the fourth ventricle. Apart from stenosis of the aqueduct, several complications are reported with shunting procedures to decompress a Dandy-Walker cyst (DWC).25–27 Common complications include posterior fossa subdural hematomas, cranial nerve palsy with malpositioning of a shunt catheter, CSF leak, intracystic hemorrhage, and a blocked shunt.28,29 Lee et al25 reported complications related to the catheter placement due to injury to the fourth ventricle floor. One patient had a catheter tip in the brainstem. To minimize the complications resulting from catheter placement, Lee et al proposed a small 2 × 3 cm craniotomy inferior and medial to the junction of transverse and sigmoid sinuses. They also proposed that the placement of the catheter be done under ultrasound guidance. Montes et al30 advocated stereotactic transtentorial hiatus placement of a catheter. Recent studies have evaluated the role of endoscopic third ventriculostomy in selected cases of DWS.31 A 41-year-old man presented with headache, dizziness, and balance problems. He had a past history of hypertension and trauma to the head that was followed by worsening headaches 3 years ago. Neurologic examination revealed ataxia. Magnetic resonance imaging (MRI) showed a large DWC with a small cerebellum and absent/rudimentary vermis. He underwent surgery for resection of the cyst through a suboccipital craniectomy. An incision on the midline of the occipital region down to C2 was made, and under microscopic guidance an arachnoid cyst was drained completely. There was no postoperative complication, and the patient was discharged 3 days later. He had good relief of his symptoms and was able to ambulate well at the time of discharge. Two-year follow-up showed no neurologic deterioration or recurrence of symptoms other than mild headaches. It is very rare to have DWC presenting in adulthood. When there is a proximal obstruction at the cerebral aqueduct and distal obstruction at the foramina of Magendie and Luschka, the fourth ventricle is trapped and CSF production by the choroid plexuses results in progressive dilatation. The fourth ventricle can be isolated in up to 17% of children with VP shunts who have had previous intraventricular hemorrhage,32 inflammatory processes, infection, previous intraventricular hemorrhage, or neoplastic disease (carcinomatous meningitis). The pressure gradient created by lateral ventricular shunts across the aqueduct and congenital malformations can result in obstruction of the aqueduct and fourth ventricular foramina. Progressive dilatation of the fourth ventricle results from CSF production by the choroid plexus or by a ball-valve one-way mechanism through the aqueduct. CSF diversion procedures for this complicated entity include shunt placement (transcerebellar, transaqueductal, transforminal across the foramen of Magendie and the transcortical transtentorial hiatus) in various directions. Recent advances have proposed endoscopic placement of catheters/stents, aqueductoplasty, and internal shunting procedures. As in the case of a DWC, a fourth ventricular shunt alone can be placed if imaging studies demonstrate that the aqueduct is closed, or a single catheter in lateral ventricle in the case of a patent aqueduct. However, the risk of upward or downward herniation exists with both types of shunting procedures, along with failure of either system, necessitating placement of the second shunt system. Raimondi’s Y system can help in equalization of the pressures, although not without the risk of shunt malfunction. The midline transvermian and lateral transcerebellar approaches have been used for catheter placement. Intraoperative ultrasound guidance is useful in placement of the catheter inside the cyst. However, after decompression, as the brainstem and cerebellum expand, there is a risk of brainstem injury with the catheter tip and resultant focal neurologic deficits.32 An enlarged fourth ventricle can be tapped from supratentorial entry either by the transtentorial hiatus route or the transventricular transaqueductal method.30,33 Regardless of the approach, complications and revision rates remain high.34,35 The transventricular transaqueductal approach is attractive because the catheter fenestrations can be fashioned as to drain both the lateral and fourth ventricle simultaneously. This also would synchronize the pressures inside these compartments. The transtentorial hiatus approach is appropriate for those large cysts that reach cranially across the tentorial hiatus into the supratentorial compartment. Both of these techniques require interactive digital imaging techniques and/or endoscopy. Endoscopic techniques help in opening up the aqueduct by performing aqueductoplasty, placement of stenting along with third ventriculostomy, or shunt placement.36 The reported complication rate in this series was 25%. Third ventriculostomy or aqueductoplasty alone without a shunt also had failures and complications.37 Aqueductoplasty is reported to have reclosure, requiring additional treatment.36,38,39 Stents also have been used in small numbers, and, like shunts, they are vulnerable to infection, migration, and obstruction. The management results and complications of a trapped fourth ventricle are similar to those of a DWC, although with a much wider range. Tumors of the central nervous system (CNS) are the most common solid neoplasms found in children, and the majority of these tumors are infratentorial. The proximity to the fourth ventricle and thus to the CSF pathways predisposes these children to the development of obstructive hydrocephalus.40,41 At some point, these children require a CSF diversion procedure during the course of their illness.42–44 Since the report published by Abraham and Chandy describing ventriculoatrial (VA) shunts in the management of posterior fossa (PF) tumors with hydrocephalus, many centers utilized either VP or VA shunts to reduce intracranial hypertension in PF tumors with hydrocephalus.45 Because VA or VP shunts are not devoid of problems, or more appropriately have high rates of malfunction and revisions, the routine placement of shunts has come under criticism. Major complications, including shunt malfunction, infection, migration, tumor hemorrhage, tentorial herniation, and a variety of abdominal complications, necessitated a review of the indications for routine shunt surgery in PF tumors.46–49 The American Society for Pediatric Neurosurgery performed a survey in 1985 on precraniotomy shunts and found that there was no clear evidence of advantage in routine shunting before PF tumor surgery.50 A recent conference of experienced pediatric neurosurgeons reported that hydrocephalus existed in 80% of patients with PF tumors, and 25 to 30% required postoperative treatment for persisting hydrocephalus. However, there was no consensus on the way hydrocephalus should be treated before, during, or after the posterior fossa surgery.51 Undoubtedly, there is a group of patients with PF tumors requiring CSF diversion, but there are no reliable parameters to identify this group. The incidence of progressive postoperative hydrocephalus has been reported to be between 15% and 45% of patients with PF tumors, with an even higher incidence among children younger than 3 years of age.52–54 The addition of external ventricular drainage (EVD) either preoperatively or intraoperatively (continued postoperatively in some patients), endoscopic third ventriculostomy, and placement of subcutaneous reservoir have increased the available alternatives to a permanent shunting procedure.41,55–58 Culley et al52 did a retrospective analysis of 117 pediatric patients with PF tumors to identify the factors that determine the need for VP shunts. Among the various factors studied, younger age, midline tumors, subtotal tumor excision, prolonged EVD placement, cadaver dural grafts, formation of pseudomeningocele, and CSF infection were statistically significant predictors for the need for postoperative shunt placement. This study recommends total removal of tumors, especially those of the midline, with meticulous closure of the wound, prevention of postoperative CSF leak or infection, and the avoidance of cadaver dura graft for closure. Another retrospective study of 180 children with PF tumors confirmed similar factors that predispose to persistent postoperative hydrocephalus.59 Only 12 out of 180 patients (6.7%) required direct primary postoperative shunts within 6 postoperative weeks, eight patients (4.4%) were shunted due to recurrence of tumor, and eight more (4.4%) had late shunt surgery. This 15.5% shunt insertion rate appears to be lower than the 17 to 40% rate reported in the literature.44,52,58 This also suggests that a routine endoscopic third ventriculostomy may not be required in the majority of these cases. In another study, 29% of the patients with PF tumors required ventriculostomy, and 21% required a permanent shunt.60 Endoscopic third ventriculostomy is gaining popularity in the management of hydrocephalus with posterior fossa lesions. Tamburrini et al61 reported a 90% success rate with endoscopic third ventriculostomy. Morelli et al62 reported an 81% success rate in controlling hydrocephalus with endoscopic third ventriculostomy. A 2-year-old girl was referred to us with the diagnosis of pilocystic astrocytoma. She underwent resection of the tumor and placement of a ventriculoperitoneal shunt (Fig. 24.3A,B). Three years later she had a recurrence of the tumor, which was again operated on. When she was 9 years old she presented with headache and visual problems. Neurologic examination revealed no deficits. An MRI scan showed a large posterior fossa cyst. She underwent a suboccipital craniotomy and resection of the tumor. The postoperative course was uneventful and she was discharged without any new neurologic deficit. Nine months later she developed severe headaches along with nausea and vomiting. Physical and neurologic examinations were normal as before. An MRI scan showed a small cystic lesion with the possible diagnosis of recurrence. After a suboccipital craniotomy, microsurgical resection of the tumor was performed. Five-year follow-up showed no recurrence of tumor based on clinical and imaging exams (Fig. 24.3C). In general, most reports suggest that younger child with a midline tumor (medulloblastoma or ependymoma) and preoperative dilated ventricles is a candidate for preoperative CSF diversion procedure and might require a permanent shunt placement in due course. Fig. 24.3 Preoperative MRI scans in axial (A) and sagittal (B) views showing a large mixed-density lesion in the midline posterior fossa. The lesion is predominantly low density. Because of its midline position and compression of the cerebrospinal fluid (CSF) outlet, a ventriculoperitoneal shunt was placed initially. (C) Postoperative contrast-enhanced MRI reveals a residual tumor and posterior fossa pneumocephalus. The fourth ventricle is opened up. 1. Dandy WE, Blackfan KD. Internal hydrocephalus. An experimental, clinical, and pathological study. Am J Dis Child 1914;8:406–482 2. Cotugno D. De Ischiade Nervosa Commentarius. Neapoli: Apud Fratres Simonios, 1764 3. Key A, Retzius G. Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm: Samson & Wallin, 1875–1876 4. Aschoff A, Kremer P, Hashemi B, Kunze S. The scientific history of hydrocephalus and its treatment. Neurosurg Rev 1999;22:67–93, discussion 94–95 PubMed 5. Pudenz RH. The surgical treatment of hydrocephalus—an historical review. Surg Neurol 1981;15:15–26 PubMed 6. Dandy WE. Ventriculography following the injection of air into the cerebral ventricles. Ann Surg 1918;68:5–11 PubMed 7. Torkildsen A. A new palliative operation in cases of inoperable occlusion of the sylvian aqueduct. Acta Chir Scand 1939;82:117–125 8. Sutton JB. The lateral recesses of the fourth ventricle, their relation to certain cysts and tumours of the cerebellum, and to occipital meningocele. Brain 1887;9:352–361 9. Taggart JK, Walker AE. Congenital atresia of the foramen of Luschka and Magendie. Arch Neurol Psychiatry 1942;48:583–612 10. Benda CE. The Dandy-Walker syndrome or the so-called atresia of the foramen Magendie. J Neuropathol Exp Neurol 1954;22:14–29 11. Gardner WJ. Hydrodynamic factors in Dandy-Walker and Arnold-Chiari malformations. Childs Brain 1977;3:200–212 PubMed 12. Asai A, Hoffman HJ, Hendrick EB, Humphreys RP. Dandy-Walker syndrome: experience at the Hospital for Sick Children, Toronto. Pediatr Neurosci 1989;15:66–73 PubMed 13. Domingo Z, Peter J. Midline developmental abnormalities of the posterior fossa: correlation of classification with outcome. Pediatr Neurosurg 1996;24:111–118 PubMed 14. Dandy WE. The diagnosis and treatment of hydrocephalus due to occlusions of the foramina of Magendie and Luschka. Surg Gynecol Obstet 1921;32:112–124 15. Sahs AL. Congenital anomaly of the cerebellar vermis. Arch Pathol (Chic) 1941;32:52–63 16. Hirsch JF, Pierre-Kahn A, Renier D, Sainte-Rose C, Hoppe-Hirsch E. The Dandy-Walker malformation. A review of 40 cases. J Neurosurg 1984; 61:515–522 PubMed 17. Osenbach RK, Menezes AH. Diagnosis and management of the Dandy-Walker malformation: 30 years of experience. Pediatr Neurosurg 1992; 18:179–189 PubMed 18. Carmel PW, Antunes JL, Hilal SK, Gold AP. Dandy-Walker syndrome: clinico-pathological features and re-evaluation of modes of treatment. Surg Neurol 1977;8:132–138 PubMed 19. Raimondi AJ, Samuelson G, Yarzagaray L, Norton T. Atresia of the foramina of Luschka and Magendie: the Dandy-Walker cyst. J Neurosurg 1969;31:202–216 PubMed 20. Sawaya R, McLaurin RL. Dandy-Walker syndrome. Clinical analysis of 23 cases. J Neurosurg 1981;55:89–98 PubMed 21. Udvarhelyi GB, Epstein MH. The so-called Dandy-Walker syndrome: analysis of 12 operated cases. Childs Brain 1975;1:158–182 PubMed 22. Maria BL, Zinreich SJ, Carson BC, Rosenbaum AE, Freeman JM. Dandy-Walker syndrome revisited. Pediatr Neurosci 1987;13:45–51 PubMed 23. Foltz EL, Shurtleff DB. Conversion of communicating hydrocephalus to stenosis or occlusion of the aqueduct during ventricular shunt. J Neurosurg 1966;24:520–529 PubMed 24. Hawkins JC III, Hoffman HJ, Humphreys RP. Isolated fourth ventricle as a complication of ventricular shunting. Report of three cases. J Neurosurg 1978;49:910–913 PubMed 25. Lee M, Leahu D, Weiner HL, Abbott R, Wisoff JH, Epstein FJ. Complications of fourth-ventricular shunts. Pediatr Neurosurg 1995;22:309– 313, discussion 314 PubMed 26. McLone DG, Naidich TP, Cunningham T. Posterior fossa cysts: management and outcome. Concepts Pediatr Neurosurg 1987;7:134–137 27. Bindal AK, Storrs BB, McLone DG. Management of the Dandy-Walker syndrome. Pediatr Neurosurg 1990-1991-1991;16:163–169 PubMed 28. Villavicencio AT, Wellons JC III, George TM. Avoiding complicated shunt systems by open fenestration of symptomatic fourth ventricular cysts associated with hydrocephalus. Pediatr Neurosurg 1998;29:314–319 PubMed 29. Eder HG, Leber KA, Gruber W. Complications after shunting isolated IV ventricles. Childs Nerv Syst 1997;13:13–16 PubMed 30. Montes JL, Clarke DB, Farmer JP. Stereotactic transtentorial hiatus ventriculoperitoneal shunting for the sequestered fourth ventricle. Technical note. J Neurosurg 1994;80:759–761 PubMed 31. Mohanty A, Biswas A, Satish S, Praharaj SS, Sastry KV. Treatment options for Dandy-Walker malformation. J Neurosurg 2006;105(5, Suppl): 348–356 PubMed 32. Coker SB, Anderson CL. Occluded fourth ventricle after multiple shunt revisions for hydrocephalus. Pediatrics 1989;83:981–985 PubMed 33. Harter DH. Management strategies for treatment of the trapped fourth ventricle. Childs Nerv Syst 2004;20:710–716 PubMed 34. Chai WX. Long-term results of fourth ventriculo-cisternostomy in complex versus simplex atresias of the fourth ventricle outlets. Acta Neurochir (Wien) 1995;134:27–34 PubMed 35. Dollo C, Kanner A, Siomin V, Ben-Sira L, Sivan J, Constantini S. Outlet fenestration for isolated fourth ventricle with and without an internal shunt. Childs Nerv Syst 2001;17:483–486 PubMed 36. Teo C, Burson T, Misra S. Endoscopic treatment of the trapped fourth ventricle. Neurosurgery 1999;44:1257–1261, discussion 1261–1262 PubMed 37. Mohanty A. Endoscopic third ventriculostomy with cystoventricular stent placement in the management of dandy-walker malformation: technical case report of three patients. Neurosurgery 2003;53:1223– 1228, discussion 1228–1229 PubMed 38. Hayashi N, Hamada H, Hirashima Y, Kurimoto M, Takaku A, Endo S. Clinical features in patients requiring reoperation after failed endoscopic procedures for hydrocephalus. Minim Invasive Neurosurg 2000; 43:181–186 PubMed 39. Shin M, Morita A, Asano S, Ueki K, Kirino T. Neuroendoscopic aqueductal stent placement procedure for isolated fourth ventricle after ventricular shunt placement. Case report. J Neurosurg 2000;92:1036–1039 PubMed 40. Serlo W, Fernell E, Heikkinen E, Anderson H, von Wendt L. Functions and complications of shunts in different etiologies of childhood hydrocephalus. Childs Nerv Syst 1990;6:92–94 PubMed 41. Albright L, Reigel DH. Management of hydrocephalus secondary to posterior fossa tumors. J Neurosurg 1977;46:52–55 PubMed 42. Dias MS, Albright AL. Management of hydrocephalus complicating childhood posterior fossa tumors. Pediatr Neurosci 1989;15:283–289, discussion 290 PubMed 43. Park TS, Hoffman HJ, Hendrick EB, Humphreys RP, Becker LE. Medulloblastoma: clinical presentation and management. Experience at the Hospital for Sick Children, Toronto, 1950–1980. J Neurosurg 1983;58: 543–552 PubMed 44. Raimondi AJ, Tomita T. Hydrocephalus and infratentorial tumors. Incidence, clinical picture, and treatment. J Neurosurg 1981;55:174–182 PubMed 45. Abraham J, Chandy J. Ventriculo-atrial shunt in the management of posterior-fossa tumors: preliminary report. J Neurosurg 1963;20:252– 253 PubMed 46. Davidson RI. Peritoneal bypass in the treatment of hydrocephalus: historical review and abdominal complications. J Neurol Neurosurg Psychiatry 1976;39:640–646 PubMed 47. Epstein F, Murali R. Pediatric posterior fossa tumors: hazards of the “preoperative” shunt. Neurosurgery 1978;3:348–350 PubMed 48. George R, Leibrock L, Epstein M. Long-term analysis of cerebrospinal fluid shunt infections. A 25-year experience. J Neurosurg 1979;51:804– 811 PubMed 49. McLaurin RL. Disadvantages of the preoperative shunt in posterior fossa tumors. Clin Neurosurg 1983;30:286–292 PubMed 50. McLaurin RL. On the use of pre-craniotomy shunting in the management of posterior fossa tumors in children. Concepts Paediatr Neurosurg 1985;6:1–5 51. Schijman E, Peter JC, Rekate HL, Sgouros S, Wong TT. Management of hydrocephalus in posterior fossa tumors: how, what, when? Childs Nerv Syst 2004;20:192–194 PubMed 52. Culley DJ, Berger MS, Shaw D, Geyer R. An analysis of factors determining the need for ventriculoperitoneal shunts after posterior fossa tumor surgery in children. Neurosurgery 1994;34:402–407, discussion 407– 408 PubMed 53. Kumar V, Phipps K, Harkness W, Hayward RD. Ventriculo-peritoneal shunt requirement in children with posterior fossa tumours: an 11-year audit. Br J Neurosurg 1996;10:467–470 PubMed 54. Tamburrini G, Di Rocco C, Caldarelli M, Di Rocco F, Sabatino G, Koutzoglou M. Postoperative third ventriculostomy in children with posterior cranial fossa tumors. Childs Nerv Syst 2003;19:691–692 55. Schmid UD, Seiler RW. Management of obstructive hydrocephalus secondary to posterior fossa tumors by steroids and subcutaneous ventricular catheter reservoir. J Neurosurg 1986;65:649–653 PubMed 56. Rappaport ZH, Shalit MN. Perioperative external ventricular drainage in obstructive hydrocephalus secondary to infratentorial brain tumours. Acta Neurochir (Wien) 1989;96:118–121 PubMed 57. Feng H, Huang G, Liao X, et al. Endoscopic third ventriculostomy in the management of obstructive hydrocephalus: an outcome analysis. J Neurosurg 2004;100:626–633 PubMed 58. Sainte-Rose C, Cinalli G, Roux FE, et al. Management of hydrocephalus in pediatric patients with posterior fossa tumors: the role of endoscopic third ventriculostomy. J Neurosurg 2001;95:791–797 PubMed 59. Bognár L, Borgulya G, Benke P, Madarassy G. Analysis of CSF shunting procedure requirement in children with posterior fossa tumors. Childs Nerv Syst 2003;19:332–336 PubMed 60. Mangubat EZ, Chan M, Ruland S, Roitberg BZ. Hydrocephalus in posterior fossa lesions: ventriculostomy and permanent shunt rates by diagnosis. Neurol Res 2009;31:668–673 PubMed 61. Tamburrini G, Pettorini BL, Massimi L, Caldarelli M, Di Rocco C. Endoscopic third ventriculostomy: the best option in the treatment of persistent hydrocephalus after posterior cranial fossa tumour removal? Childs Nerv Syst 2008;24:1405–1412 PubMed 62. Morelli D, Pirotte B, Lubansu A, et al. Persistent hydrocephalus after early surgical management of posterior fossa tumors in children: is routine preoperative endoscopic third ventriculostomy justified? J Neurosurg 2005;103(3, Suppl):247–252 PubMed
Shunt Surgery for Posterior Fossa Lesions
♦ Dandy-Walker Cyst
♦ Case Illustration
♦ Trapped Fourth Ventricle
♦ Neoplastic Lesions of Posterior Fossa
♦ Case Illustration
♦ Conclusion
References
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








