23
Posterior Fossa Arteriovenous Malformations and Cavernous Angiomas
Ramachandra P. Tummala, Mustafa K. Başkaya, and Roberto C. Heros
♦ General Surgical Strategies
The planning of a surgical approach in the posterior fossa is dependent on several factors. The shortest path from the surface of the patient to the epicenter of the lesion is determined. The margin of the lesion that is closest to a pial or ependymal surface must be identified to avoid or minimize the amount of dissection through normal tissue. After determining which of the cerebellar surfaces (tentorial, petrous, or suboccipital) offers the maximal visualization of the lesion, a group of approaches is chosen as potential candidates. A final surgical plan is chosen based on the surgeon’s preference and familiarity with a certain approach and a thorough knowledge of the regional anatomy. Of course, the most direct path to the lesion may not be the safest or most practical, and compromises in the approach may be necessary. One additional critical factor that affects the choice of approach in arteriovenous malformation (AVM) surgery is the location of arterial feeders. This is in contrast to cavernous malformation surgery in which the critical factor in the approach is the surface representation of the lesion.
Lesions of the inferior vermis, cerebellar tonsils, medial cerebellar hemispheres, and brainstem that extend through the floor of the fourth ventricle are best approached through a midline suboccipital craniotomy. More laterally situated cerebellar hemispheric lesions can be approached through a paramedian variant (Fig. 23.1). The patient is positioned prone on large chest rolls with the neck flexed to expose the foramen magnum and posterior arch of C1. The patient is placed in a reverse Trendelenburg position to bring the head parallel to the floor. A craniotomy can be carried inferiorly to include the foramen magnum depending on the location of the lesion. After opening the dura in a Y-shaped fashion, cerebellar relaxation is obtained by releasing cerebrospinal fluid (CSF) from the cisterna magna.
Lesions involving the tentorial surface of the cerebellum, tectal plate, or the pineal region can be reached through two main approaches. The supracerebellar, infratentorial approach provides a tangential view of the rostral vermis, the quadrangular lobules, and the superior semilunar lobule (Fig. 23.2). This approach can be performed with the patient in either the sitting or the Concorde position. Although precautions must be taken to detect and treat air embolism, we prefer the sitting position because the effects of gravity cause the cerebellum to fall inferiorly. Another potential disadvantage of this position is its initial awkwardness and long working distance to the lesion, given the fact that the operative microscope must be placed between the surgeon’s head and the patient. The body of the microscope is centered between the surgeon’s arms as opposed to the more natural position of the surgeon’s arms underneath the microscope. These conditions may increase fatigue in the upper extremities of the surgeon. We believe these inconveniences are offset by the increased exposure obtained from the effects of gravity on the cerebellum. We obtain additional exposure by routinely extending the craniotomy 2 cm above the torcular and transverse sinuses, allowing for maximal retraction of the superior leaf of dura based along the transverse sinuses. A paramedian or more lateral variant of this approach offers a more direct view of the superior cerebellar peduncle and is optimal for unilateral lesions between the trigeminal nerve and lateral border of the tectal plate.
If the cerebellar malformation is located more anteriorly and remains unilateral, the occipital transtentorial approach should be considered; we prefer this approach for lesions supplied by the superior cerebellar artery and its branches from one side only (Fig. 23.3). This approach is also satisfactory for lesions in the retromesencephalic region that extend inferiorly along the cerebellomesencephalic fissure. The patient is secured in the lateral position with the ipsilateral occipital lobe down. This positioning allows the occipital lobe to fall away from the falx, and the additional use of lumbar drainage results in minimal brain retraction. The craniotomy crosses the sagittal and transverse sinuses as in the supracerebellar, infratentorial approach. We divide the tentorium parallel to the straight sinus to expose the tentorial surface of the cerebellar hemispheres and the rostral vermis. Great care must be used to dissect through the thick layer of arachnoid overlying the vein of Galen and its tributaries. These veins obscure the contralateral structures. The occipital lobe has little tolerance for retraction, and despite the precautions taken to minimize the retraction, visual field deficits remain a serious potential consequence of this operation.
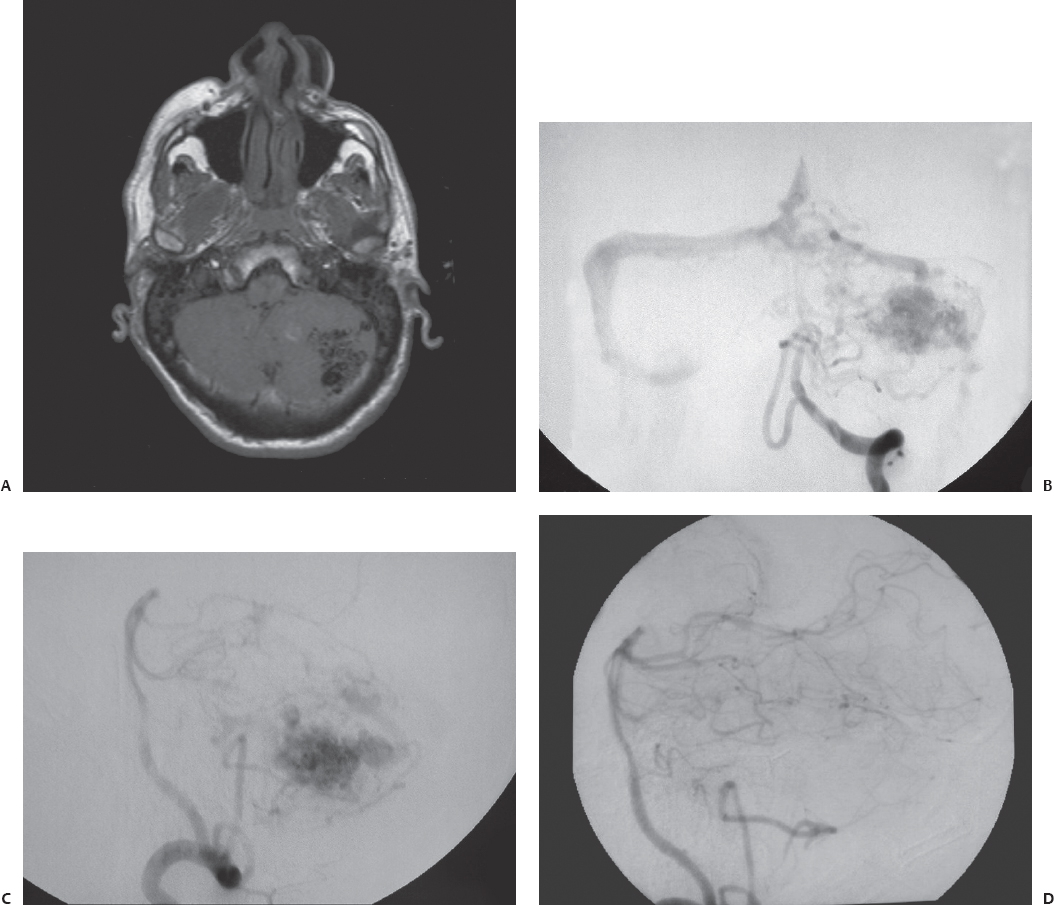
Fig. 23.1 (A) Axial T1-weighted magnetic resonance imaging (MRI) shows a left cerebellar arteriovenous malformation (AVM) in a 42-year-old man who presented initially with lethargy and ataxia from hemorrhage. Anteroposterior (B) and lateral (C) views of the left vertebral injection during cerebral angiography demonstrated the AVM. After recovering from the hemorrhage and the associated hydrocephalus, the AVM was resected through a left paramedian suboccipital craniotomy. (D) Postoperative angiography confirmed complete resection of the lesion.
The other frequent approach for posterior fossa AVMs and cavernous malformations is the retrosigmoid approach (also referred to as retromastoid and lateral suboccipital approaches). Originally designed for cerebellopontine angle lesions, this approach can also be applied to vascular malformations of the petrous surface of the cerebellum and the lateral pons. We prefer the lateral position with the head parallel to the floor. Flexion of the neck provides increased visualization of inferiorly situated lesions and increases the working space above the shoulder. We supplement a conservative retrosigmoid craniotomy with a craniectomy to skeletonize the sigmoid sinus up to its junction with the transverse sinus. The dura is then opened in either a Y or a lambda (λ) pattern. The release of CSF from the cisterna magna or from the lateral cerebellomedullary cistern provides relaxation of the cerebellum and facilitates retraction to visualize the petrous surface. The middle cerebellar peduncle is also seen through this tangential exposure.
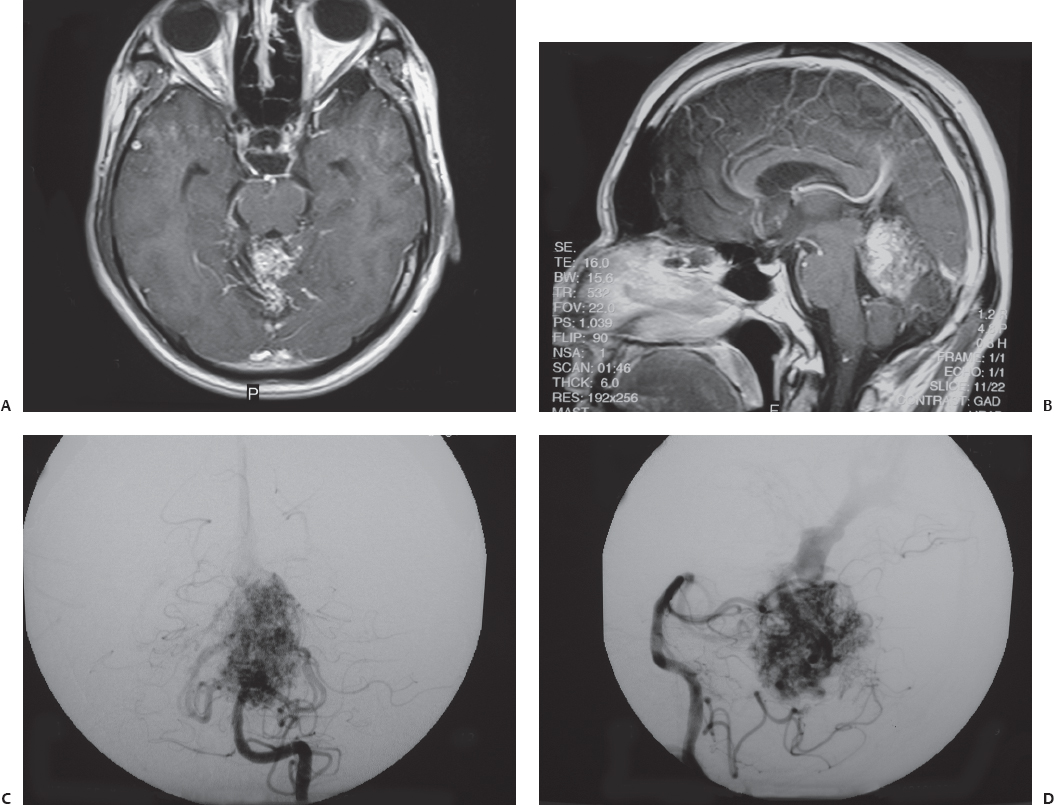
Fig. 23.2 Axial (A) and sagittal (B) contrasted T1-weighted MRI of a 33-year-old man who was obtunded from cerebellar and intraventricular hemorrhages. During cerebral angiography, anteroposterior (C) and lateral (D) views of the left vertebral artery injection confirmed a superior vermian AVM. After the patient improved neurologically, this lesion was resected through a supracerebellar, infratentorial approach with the patient in the sitting position.
For more lateral approaches that minimize cerebellar retraction, the posterior temporal bone is resected to varying degrees. This family of presigmoid approaches includes the retrolabyrinthine, translabyrinthine, transcochlear, and transotic.1 For AVMs, we have never used more than a simple presigmoid, retrolabyrinthine approach combined with a small subtemporal craniotomy and division of the superior petrosal sinus and the tentorium all the way through the incisura. This combined approach enables the safe division of the tentorium, preserving the temporal lobe draining veins, and provides a more direct view of the anterior and lateral surface of the brainstem and the region of the superior cerebellar peduncle. Finally, lesions involving the lateral and posterolateral medulla as well as the anterior cerebellar tonsils are best approached by the far lateral transcondylar approach.2
♦ Microsurgery for Posterior Fossa Arteriovenous Malformations
Technical Points
Arteriovenous malformations of the posterior fossa represent approximately 20% of all intracranial AVMs.3 Brainstem and cerebellar AVMs were considered historically to be similar entities because of their common vascular supplies. It is now clear that AVMs of the cerebellum and brainstem have distinct natural histories and different presentations.4,5 For the present discussion, we shall consider them separately.

Fig. 23.3 Coronal contrasted T1-weighted (A) and axial T2-weighted (B) MRI of a 46-year-old woman who presented with progressive ataxia. The imaging characteristics are consistent with a cavernous malformation involving the anterior tentorial surface of the left cerebellar hemisphere. Because of its anterior, unilateral location, the lesion was resected through an occipital, transtentorial approach with the left side down. The patient developed a right-sided hemianopsia postoperatively, which resolved within 2 weeks.
Cerebellar AVMs have been classified by their location in the cerebellar hemispheres, tonsils, and vermis. Brainstem AVMs have been categorized as superficial or deep.6 Superficial lesions are pial or subpial and derive all their arterial feeders from circumferential arteries coursing around the brainstem with no perforating arterial supply. Reports of successful resection of brainstem AVMs have been limited to these superficial lesions.4,7 Deep AVMs are surrounded by brainstem parenchyma and are supplied by perforating arteries; this makes them inoperable for all practical purposes, and we shall not discuss them further.
As implied earlier, one of the key considerations in planning the surgical approach to any AVM is the early control of the arterial feeding pedicles. In general, one should respect the principle of taking the arterial supply only at the point where it reaches the malformation to avoid excessive parenchymal damage from occlusion of branches to normal brain before the feeder reaches the AVM. However, at times, “safety” supersedes “finesse,” and there are instances where we occlude the arterial supply to cerebellar AVMs as these feeders enter the cerebellum. This is particularly true with large AVMs involving one cerebellar hemisphere. In general, we have found that it is safe to occlude superior cerebellar branches that are presumed to go to a large cerebellar hemispheric AVM as they enter the cerebellum after coursing around the brainstem and giving all the important perforators to the brainstem. This arterial supply can be safely reached by either the supracerebellar, infratentorial approach or the occipital, transtentorial approach in smaller lesions that have unilateral supply, as indicated above. For unilateral large cerebellar hemispheric lesions that reach the petrous surface, we prefer the retrosigmoid approach, which can be extended superior to essentially a far lateral, supracerebellar, infratentorial approach and inferiorly to a far lateral, suboccipital, transcondylar approach. With these extensions, one can control all three main arterial supplies to a unilateral cerebellar lesion; the superior cerebellar supply can be controlled by going over the superior lateral surface of the cerebellum. The anterior inferior cerebellar supply can be controlled in the cerebellopontine angle, and in general we have found it safe to take anterior inferior cerebellar branches once they have passed behind the seventh and eighth cranial nerve complex and the region of the foramen of Luschka to enter the cerebellar hemisphere; all the important branches to the brainstem have been given off by this time. With the far lateral suboccipital extension, and particularly by extending the craniotomy to the midline in the area of the foramen magnum, all of the posterior inferior cerebellar supply can be controlled. In general, we have found it safe to take any of the branches as they enter the cerebellum laterally and inferiorly, after following the posterior inferior cerebellar artery (PICA) between the tonsils to the choroidal point, one can sacrifice the branches that come back as they enter the cerebellar hemisphere in cases of large AVMs. Again, all the important branches of the PICA to the brainstem and to the deep gray matter of the cerebellum are given off before or at the choroidal point.
To reiterate, we do not recommend surgery for intrinsic AVMs of the brainstem fed by perforating vessels. The only AVMs we have approached are very small superficial lesions that are mostly pial or subpial and where the arterial supply is presumed to come around and not through the brainstem. Even when these conditions are met, we still find these lesions very difficult to treat, and on more than one occasion we have abandoned the attempt after not being able to identify clearly which small arterial branches go to the AVM and which go to the brainstem. Frequently, we place small temporary clips in all the feeders that we think go to the AVM and observe the vein for a significant change in color indicating dearterialization. If this does not occur, and the draining vein remains tense and red, we prefer to abandon the attempt at surgical excision and consider radiosurgery.
After controlling the main arterial supply, the AVM is usually not completely dearterialized and the vein almost always remains red. It is a matter of surgical judgment for the surgeon to decide when the lesion is ready for the surgeon to begin circumferential dissection, which should proceed always in a plane as close to the malformation as possible. However, hemispheric cerebellar AVMs afford the surgeon a generous dissection plane that may be a few millimeters from the AVM. Almost invariably, deep perforating vessels keep the lesion arterialized after control of the major arterial supply and have to be controlled at the very end of the dissection. These are very fragile vessels that are difficult to coagulate and retract easily into the parenchyma, causing further damage as the surgeon attempts to occlude them. We have found Sundt microclips (Codman and Shurtleff, Raynham, MA) invaluable in controlling these small deep perforators. Most frequently, the ependyma of the ventricle needs to be reached before full control of this perforating blood supply, and it is at that time that the vein or veins finally become blue and the lesion can be removed after ligation these vessels. With brainstem AVMs, as implied above, the surgeon does not have the luxury of dissecting around the AVM into the brainstem parenchyma if the lesion is still arterialized. One simply cannot afford to produce any significant damage of the brainstem by trying to control perforating arterial supply; therefore, unless the lesion can be dearterialized completely by control of feeders on the surface of the brainstem, it is best to abandon the attempt at excision.
Intraoperative Complications
As we review our results, our most common cause of intraoperative morbidity has been what we generally classify under “poor judgment.” This essentially refers to operating on AVMs either in the brainstem or extending into the brainstem through the cerebellar peduncles. This mistake was easier to make before the days of magnetic resonance imaging (MRI) when we frequently operated on cerebellar AVMs that involved the cerebellar peduncles or AVMs that we thought were primarily in the peduncles. It was a difficult matter to discern that the AVM did not extend into the brainstem or obtain a deep perforating supply through the peduncles that had to be followed into the brainstem. Nowadays, with a good MRI, it is relatively straightforward to know when such extension exists. Deep bleeding from perforating vessels that had to be followed through the deep gray matter of the cerebellum has been another source of morbidity, although that morbidity generally consists only of cerebellar ataxia that is not a major source of functional disability, and these patients are almost always independent. On a few occasions, we have encountered significant intraoperative bleeding from beginning the dissection of the AVM before sufficient control of the arterial supply either at surgery or by preoperative embolization. Generally, we have been cautious about using preoperative embolization because of its associated significant morbidity; however, clearly there are a few instances where we wish we had used preoperative embolization because the intraoperative bleeding encountered resulted in significant disability that probably could have been avoided. The other type of significant problem that we have encountered in a few instances is cranial nerve damage with lesions that extend into the cerebellopontine angle or around the lower cranial nerves. Needless to say, with more careful microsurgical technique, these complications could have been avoided.
Postoperative Complications
Hemorrhage
The most serious complication after AVM surgery is hemorrhage from residual fragments of an AVM or from insecure hemostasis. An unrecognized small piece of residual AVM is most frequently the source of bleeding because the necessity to excise an AVM on a plane very close to its margin creates the potential for leaving behind small remnants of AVM, which represent a significant risk of hemorrhage because they are still arterialized and frequently disconnected from its venous drainage. Additionally, at the end of the resection, in the deeper portion of the AVM, the surgeon frequently has difficulty differentiating true AVM from fragile deep feeding and draining vessels. Intraoperative angiography is very useful in detecting residual portions of AVM. However, certain operative positions may pose some difficulties obtaining proper images. In these instances, the patient should undergo immediate postoperative angiography before awakening from anesthesia, and if any residual AVM is found, the patient should be taken back to surgery for resection of the remaining AVM. The exception to the need for intraoperative or immediate postoperative angiography is with simple, smaller surface AVMs when the experienced surgeon can be relatively sure that the AVM has been completely removed.
To avoid the second error, insecure hemostasis, we perform the entire procedure under normotensive blood pressure. The use of hypotension can reduce the amount of bleeding during surgical dissection but may increase the risk of postoperative hemorrhage from insecure hemostasis. After resection of the nidus, we routinely elevate the blood pressure by approximately 20 to 30 mm Hg and observe the resection cavity for 10 minutes. With this maneuver, we have encountered spontaneous bleeding within a few minutes in several patients. Needless to say, meticulous control of the blood pressure to parameters below the level at which hemostasis was achieved is essential during the wound closure, emergence from anesthesia, and the immediate postoperative period.
Venous Thrombosis
There is a theoretical risk of inducing stasis in long segments of veins where flow is suddenly interrupted after resection of high-flow AVMs. We have had one case of retrograde thrombosis and postoperative venous infarction after a straightforward resection of a high-flow AVM of the cerebellar vermis. At the end of the excision, we noticed that the markedly dilated internal cerebral veins, basal veins, and vein of Galen were relatively collapsed. The patient was comatose postoperatively, and a postoperative angiogram showed no filling of the deep venous system. The patient remained in a coma for several days but gradually began to improve to the point that he had an incomplete, but remarkable, recovery.
Common features of the previously reported cases and the one presented above are high flow to the AVM, extensive retrograde venous drainage, and frequent occlusion or stenosis of the antegrade venous drainage.8 According to a study of 33 patients in whom flow velocities and CO2 reactivities were measured, the flow velocity in the draining vessels was close to zero after removal of the AVM nidus.9 This may lead to venous thrombosis in the draining vessels because pathologic changes in the draining veins have already taken place, as a normal vein with normal endothelium is not likely to thrombose.
When faced with a high-flow AVM where the venous drainage is retrograde into normal veins that ordinarily drain parenchyma, it is desirable to reduce the flow gradually in the malformation with staged embolization or staged ligation of feeding arteries. Profound neurologic deficits due to venous thrombosis can have a much better prognosis for eventual recovery than similar neurologic deficits due to arterial occlusive disease, as in our case. An important clue that this type of complication would result in a good recovery is that the computed tomography (CT) scans may not show significant, irreversible brain damage in spite of the profound neurologic deficit during the initial postoperative period. Finally, these patients should be kept well hydrated during the intraoperative and postoperative periods to avoid further collapse of veins.
♦ Microsurgery for Posterior Fossa Cavernous Malformations
Indications
Infratentorial cavernous malformations (CMs) cause symptoms and neurologic deficits from hemorrhages, from obstructive hydrocephalus, or from mass effect from the growth of the lesion from repeated small hemorrhages. Relatively small brainstem lesions may result in significant deficits due to their critical location. Seizures are not a feature of posterior fossa lesions. Although headaches should not be a significant feature unless associated with hydrocephalus, we have seen several patients presenting with an acute headache associated with neurologic deficits. The technical points of surgery for cerebellar CMs are not significantly different from resection of supratentorial CMs. By virtue of their critical location, we shall focus here on the technical points of surgery for brainstem CMs.
The natural history of CMs is not as well defined as that of AVMs; however, an approximately 0.6 to 0.7% yearly risk of hemorrhage has been estimated for asymptomatic lesions.10,11 The rate of rehemorrhage in lesions that present with a clinically significant hemorrhage has been estimated to be approximately 4.5%.10 This rate depends on how hemorrhage is defined (i.e., by interval changes on MRI or, more strictly, by a significant clinical worsening accompanied by a clear indication of new hemorrhage on MRI). It has been suggested by several groups that brainstem CMs, which account for approximately 20% of all intracranial CMs, have a higher rate of bleeding.12–16 A large review of brainstem CMs estimated the rate of hemorrhage per year per lesion at 2.7%.16 However, this may be observational bias because brainstem lesions are more likely to present clinically when they hemorrhage compared with CMs in less eloquent areas of the brain.
The surgical resection of CMs contained within normal brainstem parenchyma carries a high morbidity. Recurrent hemorrhages may cause enlargement of the lesion and more pronounced neurologic deficits, but they rarely result in catastrophic deficits or death. As the lesion enlarges from repeated hemorrhages, it may dissect slowly through the parenchyma to the pial or ependymal surface. Once this occurs, they become more surgically accessible and obviate the need for incising the brainstem, thus carrying less surgical morbidity than their deeper counterparts.17
Technical Points
Patients with an acute brainstem hemorrhage may look severely disabled, with poor prognosis; however, considerable neurologic improvement is to be expected. Thus, the surgeon must be cautious about operating acutely under the assumption that the patient already has a major deficit, and that surgery will not worsen the condition of the patient. Frequently, in this setting, a deficit that may likely have improved spontaneously may become irreversible as a consequence of ill-advised surgery.
The general surgical techniques for posterior fossa CM resection are no different from those for supratentorial CMs. Conventional imaging may be supplemented by frameless stereotactic guidance to plan the surgical approach. We routinely monitor motor, somatosensory, and brainstem evoked potentials during the resection. The only sound surgical strategy to remove a CM is an “all-or-none” commitment, although we must emphasize that the end point with large brainstem CMs is not as clear-cut as with AVMs. In the case of the latter, bleeding usually continues until the AVM is removed completely with intraoperative angiographic confirmation. On the other hand, the surgeon may attack the interior of a brainstem cavernoma, coagulating lobules of an irregular lesion to “shrink” it away from the parenchyma. Under these circumstances, it is easy to resect a cavernous angioma incompletely, as we have done in two cases. Ideally, resection should be done by respecting the gliotic plane induced by hemorrhage. It is intuitive that the brainstem is unforgiving of spatial and technical errors.
Surgery of cavernous angiomas is very different from surgery for AVMs. The key step in AVM surgery is to occlude as much of the arterial supply to the lesion as possible before beginning resection of the lesion. In contrast, we preserve all significant (more than half a millimeter approximately) arterial or venous branches of cavernous angiomas. Our observations concur with others’ that many of these cavernous angiomas of the brainstem exist in close relationship to venous angiomas, and one of the keys to successful surgery of cavernous angiomas is full preservation of the frequently associated venous angiomas. Clearly, the cavernoma receives small arterial branches and is drained by very small venules, but, as a rule, no major arterial branches feed the lesion nor do major veins drain it. In this respect, cavernoma surgery is perhaps more similar to tumor surgery and can be likened to removal of a well-circumscribed cerebral metastasis. One can frequently work within the cavernoma because these lesions are under relatively low flow. Although this is not necessary in other locations in less eloquent brain, we frequently find it necessary in cavernomas of the brainstem, as indicated above, to get within the lesion, decompress it and gradually coagulate it away from the brain. This is very different from the strategy with cerebral AVMs.
The key to successful cavernoma surgery, then, is to avoid parenchymal damage in the trajectory toward the lesion. Because there is practically no “silent” brainstem parenchyma for the surgeon to traverse, we generally avoid surgery of deeply located brainstem cavernomas. Only when they come to the surface do we approach these lesions, and then the key is to choose an appropriate surgical approach to get us as close to the lesion as possible and to obtain an orthogonal view of the lesion. To this effect, CMs that reach the surface of the fourth ventricle are approached by a suboccipital craniotomy by either splitting the tonsils or, if they are more laterally located, through a velotonsillar approach. Whenever we operate in the floor of the fourth ventricle, we use facial nerve monitoring to be able to localize with precision the facial colliculus. Except for relatively small lesions that come to the floor of the fourth ventricle, we have been unable to remove large cavernomas of the pons through this approach without either producing a facial palsy or exacerbating a preexisting palsy. Therefore, in patients with normal facial function, we are very reluctant to use this approach, and prefer to wait until the cavernoma has already produced significant facial weakness (Fig. 23.4). For lesions that come to the surface of the posterolateral or lateral medulla or lower pons, we use the far lateral suboccipital approach. Higher pontine lesions that come to the surface laterally can be approached through a straightforward retromastoid craniectomy as used for microvascular decompression of the trigeminal nerve. More anterolateral pontine lesions can be approached through the presigmoid retrolabyrinthine exposure discussed above. For lateral mesencephalic lesions, we prefer the subtemporal transtentorial approach; however, the fourth nerve is encountered with this approach and must be preserved (Fig. 23.5). Lesions of the tectal place are best resected through the supracerebellar infratentorial exposure, an approach that can be modified to a more lateral exposure if necessary.18
Postoperative Outcomes
Because of their critical location, brainstem CMs represent a formidable neurosurgical challenge. The consensus among most neurosurgeons is that patients presenting with significant neurologic deficits should be treated surgically if the lesion comes to an “accessible” pial surface, such as the floor of the fourth ventricle or the lateral aspect of the brainstem. Patients with deeper lesions are generally monitored and surgically treated only if they exhibit severe progressive symptoms or if they develop permanent neurologic deficits that would result by default from surgery. This conservative attitude is justified because, as discussed previously, hemorrhages from these low flow lesions are rarely devastating and are usually confined by a gliotic plane, resulting in small increments of neurologic morbidity.
Several recent series have proven the feasibility of successful microsurgical resection of brainstem CMs.19–24 A retrospective analysis of 36 patients who underwent surgical management of brainstem CMs demonstrated postoperative complications including new cranial nerve deficits in 17, motor deficits in three, and new sensory complaints in 12 patients; postoperative Karnofsky Performance Scale (KPS) scores ranged from 80 to 100 compared with an average preoperative KPS score of 70.19 Outcomes for patients treated surgically and conservatively have been compared in retrospective studies. Of the patients treated surgically, approximately 84% had no or minimal deficits at follow-up. This compared favorably with the 66% of conservatively managed patients with a similar outcome.25
The encouraging results from the above series should be interpreted with caution and should not be regarded as an open invitation to resect all brainstem CMs. For example, a study comparing a group of surgical and nonsurgical patients with brainstem CMs showed that 38% of the surgical patients were disabled permanently after surgery, whereas 38% improved; none in the nonoperated group had worsened at 47-month follow-up. Only patients with multiple deficits and progressive neurologic deterioration improved with surgery.26 It is clear that successful surgical outcome depends on appropriate patient selection, optimal timing in relation to hemorrhage, careful surgical planning, meticulous surgical technique, and completeness of resection.
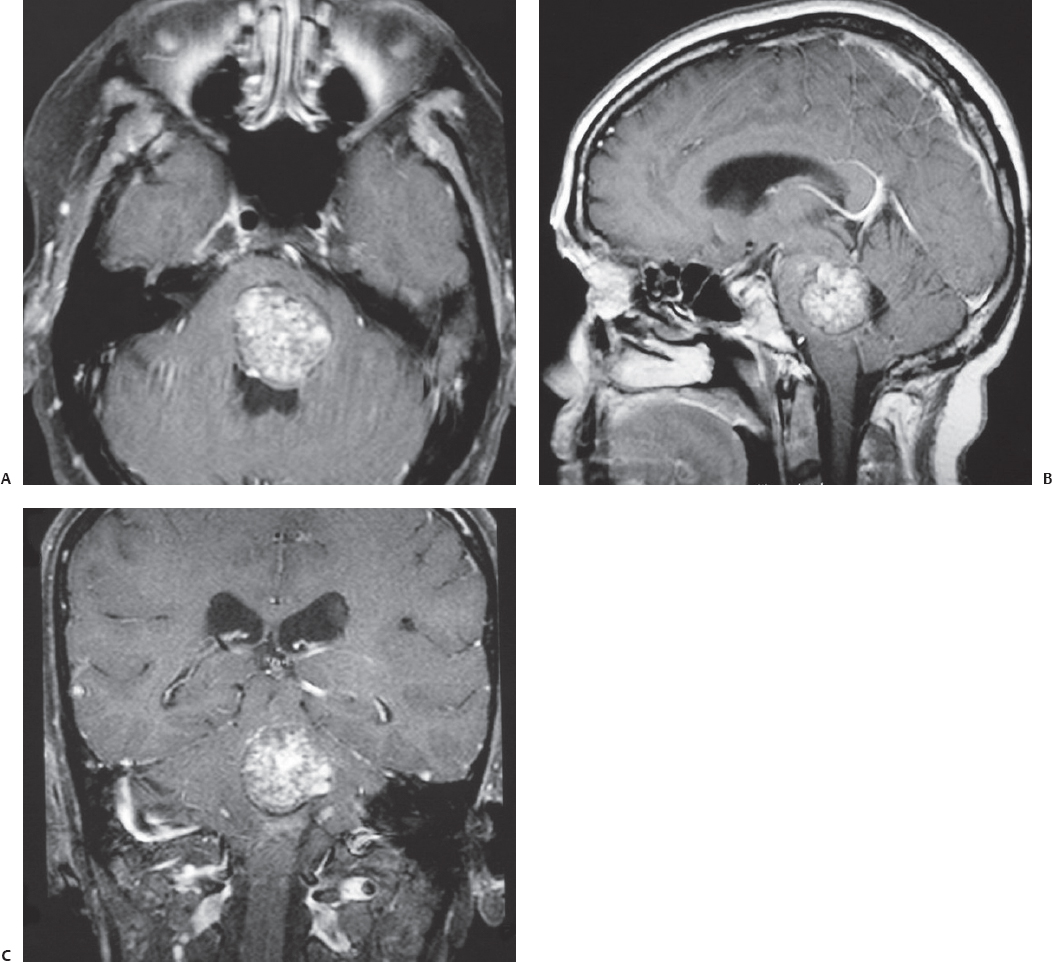
Fig. 23.4 Axial (A), sagittal (B), and coronal (C) contrasted T1-weighted MRI of a large pontine cavernous malformation in a 55-year-old woman presenting with left sixth and seventh nerve palsies and rapid worsening of ataxia and right-sided hypoesthesia. The cavernous malformation reaches the floor of the fourth ventricle. This lesion was resected completely through a midline suboccipital approach along with monitoring of the facial nerves, brainstem auditory evoked responses, somatosensory evoked potentials, and motor evoked potentials. The patient’s sixth and seventh nerve palsies remained unchanged after surgery.
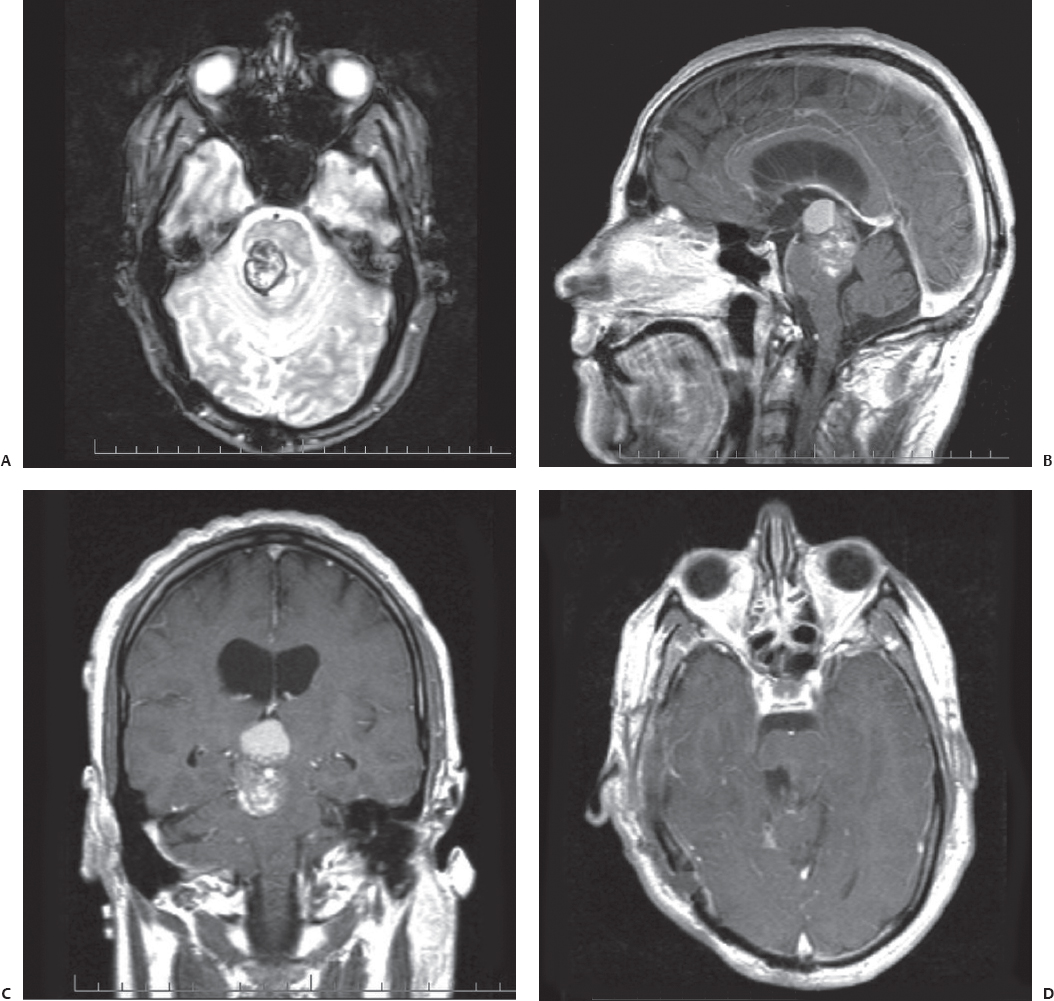
Fig. 23.5 Axial T2-weighted (A), contrasted coronal (B), and contrasted sagittal (C) MRI reveals a large right pontomesencephalic cavernous malformation in this 30-year-old man presenting with complete right third nerve palsy and left spastic hemiparesis. The patient’s symptoms progressively worsened. (D) A gross total resection was achieved through a subtemporal, transpetrous approach. The patient’s hemiparesis was worse initially after surgery but gradually returned to the preoperative level.
References
1. Morcos JJ, Heros RC, Frank DE. Microsurgical treatment of infratentorial malformations. Neurosurg Clin N Am 1999;10:441–474 PubMed
2. Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg 1986;64:559–562 PubMed
3. Drake CG, Friedman AH, Peerless SJ. Posterior fossa arteriovenous malformations. J Neurosurg 1986;64:1–10 PubMed
4. Sisti MB, Stein BM. Arteriovenous malformations of the brain stem. Neurosurg Clin N Am 1993;4:497–505 PubMed
5. Khaw AV, Mohr JP, Sciacca RR, et al. Association of infratentorial brain arteriovenous malformations with hemorrhage at initial presentation. Stroke 2004;35:660–663 PubMed
6. Batjer HH, Samson D. Arteriovenous malformations of the posterior fossa. Clinical presentation, diagnostic evaluation, and surgical treatment. J Neurosurg 1986;64:849–856 PubMed
7. Solomon RA, Stein BM. Management of arteriovenous malformations of the brain stem. J Neurosurg 1986;64:857–864 PubMed
8. Miyasaka Y, Yada K, Ohwada T, et al. Hemorrhagic venous infarction after excision of an arteriovenous malformation: case report. Neurosurgery 1991;29:265–268 PubMed
9. Hassler W, Steinmetz H. Cerebral hemodynamics in angioma patients: an intraoperative study. J Neurosurg 1987;67:822–831 PubMed
10. Kondziolka D, Lunsford LD, Kestle JRW. The natural history of cerebral cavernous malformations. J Neurosurg 1995;83:820–824 PubMed
11. Robinson JR, Awad IA, Little JR. Natural history of the cavernous angioma. J Neurosurg 1991;75:709–714 PubMed
12. Porter RW, Detwiler PW, Spetzler RF, et al. Cavernous malformations of the brainstem: experience with 100 patients. J Neurosurg 1999;90:50–58 PubMed
13. Aiba T, Tanaka R, Koike T, Kameyama S, Takeda N, Komata T. Natural history of intracranial cavernous malformations. J Neurosurg 1995;83: 56–59 PubMed
14. Isamat F, Conesa G. Cavernous angiomas of the brain stem. Neurosurg Clin N Am 1993;4:507–518 PubMed
15. Pozzati E, Acciarri N, Tognetti F, Marliani F, Giangaspero F. Growth, subsequent bleeding, and de novo appearance of cerebral cavernous angiomas. Neurosurgery 1996;38:662–669, discussion 669–670 PubMed
16. Fritschi JA, Reulen HJ, Spetzler RF, Zabramski JM. Cavernous malformations of the brain stem. A review of 139 cases. Acta Neurochir (Wien) 1994;130:35–46 PubMed
17. Wowra B, Layer G, Schad LR, et al. Three-dimensional time-of-flight MR-angiography and the surgical indication of brainstem cavernomas. Acta Neurochir (Wien) 1991;112:77–82 PubMed
18. Wen DY, Heros RC. Surgical approaches to the brain stem. Neurosurg Clin N Am 1993;4:457–468 PubMed
19. Samii M, Eghbal R, Carvalho GA, Matthies C. Surgical management of brainstem cavernomas. J Neurosurg 2001;95:825–832 PubMed
20. Sakai N, Yamada H, Tanigawara T, et al. Surgical treatment of cavernous angioma involving the brainstem and review of the literature. Acta Neurochir (Wien) 1991;113:138–143 PubMed
21. Heffez DS, Zinreich SJ, Long DM. Surgical resection of intrinsic brain stem lesions: an overview. Neurosurgery 1990;27:789–797, discussion 797–798 PubMed
22. Symon L, Jackowski A, Bills D. Surgical treatment of pontomedullary cavernomas. Br J Neurosurg 1991;5:339–347 PubMed
23. Fahlbusch R, Strauss C, Huk W, Röckelein G, Kömpf D, Ruprecht KW. Surgical removal of pontomesencephalic cavernous hemangiomas. Neurosurgery 1990;26:449–456, discussion 456–457 PubMed
24. Weil SM, Tew JM Jr. Surgical management of brain stem vascular malformations. Acta Neurochir (Wien) 1990;105:14–23 PubMed
25. Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am 1999;10:411–417 PubMed
26. Esposito P, Coulbois S, Kehrli P, et al. Place of the surgery in the management of brainstem cavernomas. Results of a multicentric study. Neurochirurgie 2003;49:5–12 PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








