Chapter 141 Monitoring and Staging Human Sleep
Abstract
History
All scientific inquiry begins with observation and description. From there it proceeds to classification based ultimately upon measurement. Thus, when Loomis and colleagues1 electroencephalographically recorded their first continuous all-night studies, they faced the daunting task of devising a system to describe sleep patterns in normal healthy human subjects. Thus sleep staging was born. In the original studies, amplified activity derived from electrodes that were placed on the scalp’s surface at several loci produced ink tracings on paper wrapped around a slowly rotating cylinder. An enormous 8-ft “drum polygraph” enabled all-night sleep recording. One electrode was located near the eye and undoubtedly detected eye movement. However, rapid eye movement (REM) sleep remained unrecognized until Aserinsky published, in part, his University of Chicago doctoral study results 17 years later.2 Aserinsky actually christened them “jerky eye movements” (JEMs) and in the first paper referred to the phenomenon as periodic ocular motility.
Perhaps it was the quirkiness of the original commercially available polygraph systems (e.g., Ofner, Beckman, and Grass) with their tendency to polarize electrodes, problematic rechargeable car battery–like systems, and aperiodic (and difficult to predict) recording interference artifacts. Or perhaps it was Loomis’s silence on the matter of eye movements during sleep. In either case, Aserinsky’s pilot work reportedly met with considerable skepticism. Ultimately, however, REM sleep’s discovery, and particularly its correlation with dreaming, altered the course of sleep research for decades.2a The near-exclusive focus on REM sleep, to the point that all other sleep states were considered simply non-REM (NREM), overshadowed substantial findings (and likely impeded progress) in other sleep research arenas (e.g., neuroendocrinology, physiology, and medicine). The spotlight on REM sleep made electrooculographic (EOG) recording de rigueur when performing sleep studies.
Meanwhile, in Lyon, France, Michel Jouvet noted postural difference during sleep in cats.3 These differences correlated with sleep state and reduced skeletal electromyographic (EMG) activity. REM sleep (and, by association, dreaming) coincided with marked hypotonia in descending alpha and gamma motor neurons. The hypotonia induced functional paralysis that was quickly ascribed the purpose of keeping the sleeper from enacting dreamed activities. This sleep state–related EMG alteration added the final compulsory recording component to the procedure now known as polysomnography (PSG).
Polysomnography, in addition to brainwave, eye movement, and muscle tone recording, can also assess respiratory, cardiac, and limb movement activity (discussed in detail in other chapters in this volume and elsewhere3a). PSG in its simplest form (including EEG, EOG, and EMG), however, provides the basic information requisite for classifying sleep state and examining sleep processes.
Electrode Placement and Application
The classic and amazingly long-lived standardized technique (i.e., the manual produced by the ad hoc committee chaired by Rechtshaffen and Kales) requires a single monopolar central-lobe scalp EEG electrode referenced to a contralateral mastoid electrode (either C3-M2 or C4-M1). This single-channel brainwave recording, when paired with right and left eye EOGs and submentalis EMG, sufficiently reveals brain, eye, and muscle activity for classifying sleep stages.4 As polysomnography evolved from a psychophysiologic research method to a clinical procedure, an occipital lead has supplemented centrally derived EEG to provide better visualization of waveforms needed to determine sleep onset and central nervous system (CNS) arousals.5,6
The American Academy of Sleep Medicine (AASM) has published a standardized manual for conducting clinical polysomnography in their accredited sleep disorders centers.7 This AASM standards manual makes recommendations for recording, scoring, and summarizing sleep stages, CNS arousals, breathing, various kinds of movement, and electrocardiographic activity. By bringing instructional guidelines for a range of techniques into a single volume, the AASM manual will strongly influence practice, particularly in North America. Researchers, however, should not feel constrained by these clinical guidelines. New discoveries and future techniques need to continue unshackled by even a de facto standard clinical practice cookbook.
Digital Recording Requirements
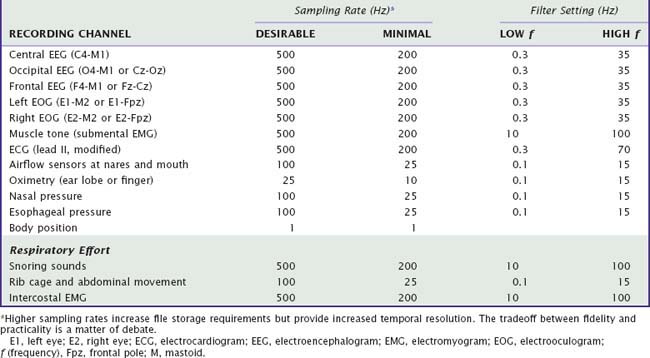
The AASM standards manual specifies minimum 12-bit representation for amplitude, providing 4096 units to represent a 2.5-volt regulated current (IREG) range, or its equivalent (Video 141-1![]() ). In this manner, even the smallest signals, exceeding the level of electrical noise, can be detected. Temporal resolution during recording depends on sampling rate and ultimately must allow accurate waveform reconstruction, provide enough data to potentially overcome frequency aliasing, and be appropriate for high- and low-pass digital filter settings. One size does not fit all: The minimum temporal resolution needed during data acquisition to meet these requirements varies for different bioelectrical signals (Table 141-1).
). In this manner, even the smallest signals, exceeding the level of electrical noise, can be detected. Temporal resolution during recording depends on sampling rate and ultimately must allow accurate waveform reconstruction, provide enough data to potentially overcome frequency aliasing, and be appropriate for high- and low-pass digital filter settings. One size does not fit all: The minimum temporal resolution needed during data acquisition to meet these requirements varies for different bioelectrical signals (Table 141-1).