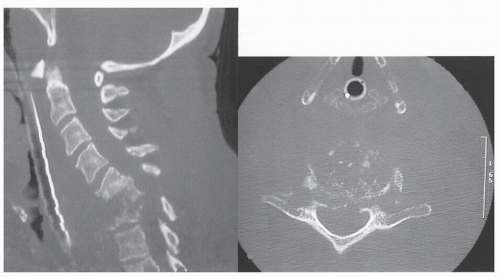
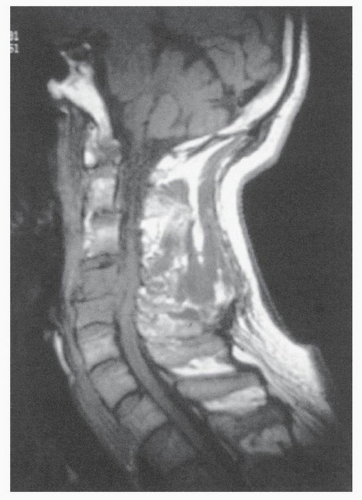
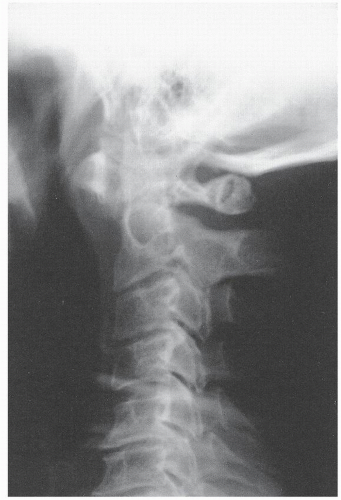
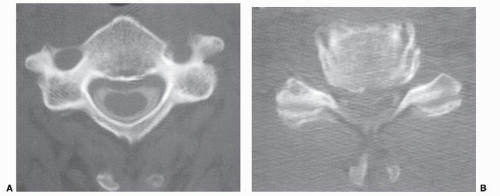
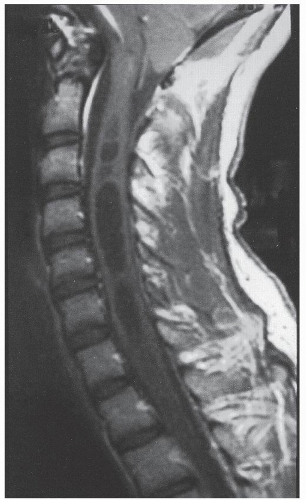
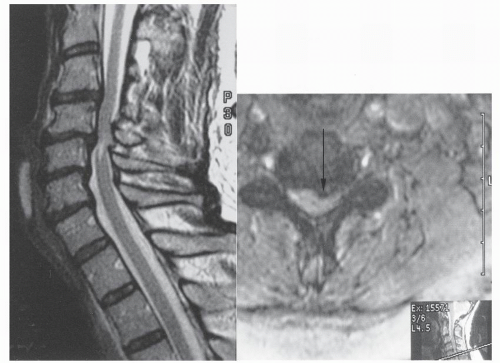
The word myelopathy comes from two Greek words: myelos meaning “spinal cord” and pathos meaning “disorder of.” A number of disease processes can disrupt the intrinsic function of the spinal cord, and many of these pathologies are treatable; it is therefore essential to find out the cause of myelopathy. Because spinal cord biopsy is rarely indicated, and many cases of myelopathy are inflammatory in nature, proper diagnosis and management rely on the patient’s history, physical examination, imaging, and cerebrospinal fluid (CSF) findings. Furthermore, in many noninfectious causes of myelopathy, early high-dose corticosteroids may alter the course of the disease, making expeditious patient evaluation imperative. A variety of imaging modalities useful in diagnosing myelopathy, as well as in determining the differential diagnosis of myelopathy, are discussed in this chapter.
CLINICAL PRESENTATION
Cervical myelopathy is common in elderly people. Cervical myelopathy has no pathognomonic signs or symptoms, and in its early course, it often has subtle and abstract symptoms.
In 1966, Crandall and Batzdorf (
1) classified patients with cervical spondylotic myelopathy into five groups, based on dominant spinal cord syndromes: (a) a syndrome due to transverse lesion; (b) a syndrome due to motor systems lesion; (c) central cord syndrome; (d) Brown-Séquard syndrome; and (e) brachialgia and cord syndromes. In transverse lesions, a complete loss of motor, sensory, and sphincter control is observed below the level of injury. The central cord syndrome, typically as a result of a hyperextension injury, is the most prevalent of the partial cord syndromes. It is characterized by bilateral motor paresis, with upper extremities affected to a greater degree than lower extremities and with distal muscle groups affected to a greater degree than proximal muscle groups. Sensory impairment and bladder dysfunction are variable (
2). In central cord syndrome, injury is typically from an inwardly bulging ligamentum flavum affecting the central gray matter and the central portions of the corticospinal and spinothalamic tracts. The prognosis for patients with the central cord syndrome is quite variable, depending on the degree of injury. Of patients younger than 50 years of age, more than 80% regain bladder continence and about 90% return to ambulatory status. Of those older than 50 years of age, only 30% regain bladder function, with about 50% regaining ambulation (
3).
Brown-Séquard syndrome, first described in 1846, results from an anatomic or functional hemisection of the spinal cord. The syndrome in its pure form is characterized by ipsilateral loss of motor function, proprioception, and vibration sense, with contralateral loss of pain and temperature sensation below the level of injury. Because fibers associated with the lateral spinothalamic tract ascend or descend one to two cord segments before crossing to the contralateral side, ipsilateral pain and temperature loss may be noted one or two segments above the lesion. Brown-Séquard syndrome has the best prognosis of any of the incomplete spinal cord syndromes, with 80% to 90% of patients regaining bowel and bladder function, 75% regaining ambulatory status, and 70% becoming independent in their activities of daily living (
3).
The anterior cord syndrome is characterized by loss of motor function, pain, and crude touch below the level of the lesion, with preservation of posterior column function, including touch, position, and vibratory sensation. The anterior spinal syndrome is most commonly reported after aortic surgery; it has also been reported after severe hypotension, infection, myocardial infarction, and aortic angiography and from a cervical hyperflexion injury resulting in a spinal cord contusion (
4). Functional recovery is variable, with most improvement made in the first 24 hours, but little improvement thereafter. Overall, about 10% to 20% of patients with this syndrome regain some muscle function; however, they have little strength or coordination (
3).
In 1985, Ferguson and Caplan (
5) defined clinical syndromes in four categories on the basis of neurologic deficits due to cervical spondylosis: (a) lateral or radicular syndrome, (b) medial or spinal syndrome, (c) combined medial-lateral syndromes, and (d) vascular syndromes.
The lateral syndrome is due to nerve root pathology, most commonly a result of compression. Clinical signs and symptoms of spinal cord compression are absent. The medial syndrome is a manifestation of a spinal cord abnormality. It has variable clinical signs that depend on the anatomic location of the pathologic factor and on the severity of its effect on the spinal cord. Combined syndromes have evidence of both nerve root and spinal cord involvement. The vascular syndrome is described as a sudden painless myelopathy, frequently in the absence of trauma that is usually associated with unimpressive imaging studies.
Generally, cervical myelopathy presents with a subtle gait disturbance, followed by upper extremity involvement (
6). Myelopathic gait may appear broad based, spastic, and with loss of smooth rhythmic motion. Sudarsky and Ronthal (
7) reported that cervical myelopathy was the most common cause of a gait disorder, accounting for 16% of elderly patients with undiagnosed gait disorders. Bowel and bladder dysfunction may occur in cervical myelopathy and are manifestations of upper motor neuron disease. The rate of bowel and bladder dysfunction in patients with previously diagnosed myelopathies is reported to be between 15% and 20% each (
8). In the series by Lunsford et al. (
6), sphincter disturbance was observed in 49% of patients. Autonomic dysfunction, such as dysreflexia and hypotension, is not very common in cervical myelopathy.
PHYSICAL EXAMINATION
The physical findings in cervical myelopathy may vary greatly from patient to patient, depending on factors such as the level of and extent of compression, duration of compression, span of segments compressed, and aggravating factors. On motor examination, both upper and lower motor neuron disturbances are observed. Generally, the lower motor neuron is involved at the level of the clinically expressed lesion, such as a site of stenosis or syrinx. Upper motor neuron involvement is observed below the site of the lesion. Therefore, in cervical myelopathy, the lower extremities express the upper motor neuron lesion, whereas the upper extremities may express both, depending on the level and nature of compression.
In cervical myelopathy, the sensory examination generally demonstrates involvement of both upper and lower extremities. There is variability in presentation. A patient may experience loss of pain and temperature, proprioception, and vibration sense below the level of the lesion. However, touch sensation is usually preserved. Involvement of a posterior column produces ipsilateral position and vibratory sense disturbance, whereas spinothalamic tract involvement produces a contralateral pain and temperature disturbance at several levels below the site of compression. If nerve roots are involved, radicular symptoms, such as numbness and tingling, are experienced in the appropriate dermatomal distribution.
Paralleling the motor examination, reflexes are generally hyperreflexic below the level of the lesion and hyporeflexic at the level of lesion. In cervical myelopathy, involvement of the upper motor neuron is also characterized by the presence of pathologic reflexes, such as the Babinski reflex in the lower extremity and the Hoffman reflex in the upper extremity. In addition, clonus may be present in the lower extremities. Lhermitte sign (a generalized electric shock sensation associated with neck flexion or extension), a classic sign of cervical myelopathy, may be present.
In the series by Lunsford et al. (
6) of 32 patients with cervical spondylotic myelopathy, 59% of patients had purely myelopathic symptoms and 41% had combined myelopathic and radicular syndromes. Corticospinal tract dysfunction was noted most frequently with motor weakness in 58% of patients. Lower extremity hyperreflexia and the Babinski response were noted in 87% and 54% of cases, respectively, whereas the Hoffman reflex was noted in 13% of cases, and Lhermitte sign was observed in only two patients (6%).
CLASSIFICATION
Nurick devised a classification system for myelopathy based on severity of gait abnormalities (
9). His scale involves a grading system of 0 to 5 for progressively worsening myelopathic gait (
Table 16.1). The Japanese Orthopaedic Association devised an objective assessment scale to quantitate severity of the spondylotic myelopathy (
10,
11 and
12). This scale encompasses four categories: motor function of both the upper and lower extremities, sensory changes in the trunk and extremities, and bladder function (
Table 16.2). This scale assesses the more global effects of myelopathy. A score of less than 7 indicates severe myelopathy, a score between 8 and 12 indicates moderate myelopathy, and a score above 13 indicates mild myelopathy.