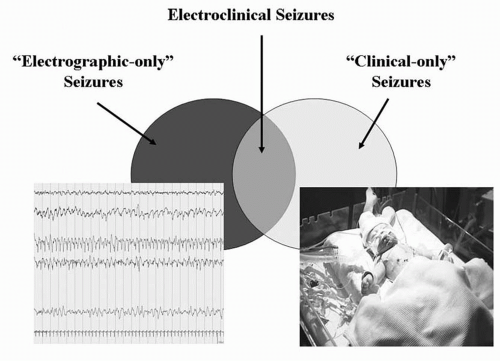
Incidence
The incidence of seizures in the first 28 days of life, one of the highest risk periods for seizures in humans, ranges between 1% and 5%. Depending on the methodology used, seizures occur at a rate of 1.5 to 5.5 per 1000 neonates (
1,
2,
3,
4,
5,
6,
7), most within the first week of life (
4). Incidence varies with specific risk factors. Lanska and colleagues (
4) reported the incidence of seizures in all neonates to be 3.5 per 1000, but 57.5 per 1000 in very-low-birth-weight (<1500 g) infants, 4.4 per 1000 in low-birth-weight (1500-2499 g) infants, and 2.8 per 1000 in normal-birthweight (2500-3999 g) infants. Scher and colleagues (
8,
9) described seizures in 3.9% of neonates younger than 30 weeks conceptional age and in 1.5% of those older than 30 conceptional weeks.
The human newborn is especially vulnerable to a wide range of toxic or metabolic conditions. Sepsis, meningitis, hypoxic-ischemic encephalopathy (HIE), hypoglycemia, and hyperbilirubinemia are capable of eliciting seizures. This may explain, in part, the frequent occurrence of braindamaging events in the first 30 days of life. However, the neonatal brain itself may be especially prone to seizures when injured.
One suspected mechanism of enhanced seizure susceptibility in the newborn is the relative imbalance between inhibition and excitation. Compared with more mature brains, the neonatal brain has delayed maturation of inhibitory circuits and precocious maturation of excitatory circuits (
10). This “imbalance” reflects a desirable and natural aspect of early central nervous system (CNS) development characterized by exuberant growth of excitatory synapses (
10) coupled with activity-dependent pruning necessary for the prodigious rate of novel learning that faces all neonates. Moreover, according to studies in the neonatal rat, γ-aminobutyric acid (GABA) (the chief inhibitory neurotransmitter of the mature brain) may exert paradoxically
excitatory effects in early CNS development (
11,
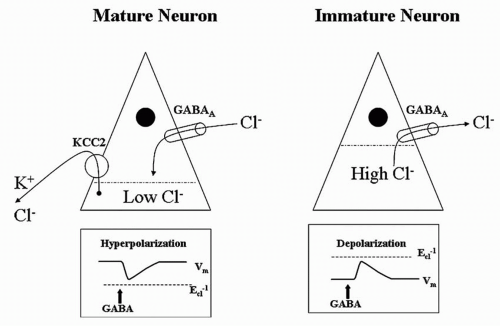
12). A developmentally dependent potassium chloride channel (KCC2) does not reach mature proportions in the rat hippocampus until the 15th postnatal day (
Fig. 32.2). This age-dependent channel pumps potassium chloride from the interior of the neuron, reducing intracellular chloride levels. Thus, when the ligand-dependent GABA receptor is opened, extracellular chloride follows its electrochemical gradient into the neuron and hyperpolarizes it as expected. However, before the appearance of KCC2, the intracellular concentration of chloride is high, exceeding that of the extracellular space. In the immature rat, activation of the GABA receptor allows chloride to run along its electrochemical gradient out of the neuron, paradoxically depolarizing it (
13).
Although neonatal seizures most commonly result from an underlying acute illness, some are reversible, the outward sign of a treatable condition. For example, the presence of hypocalcemia, hypomagnesemia, hypoglycemia, pyridoxine deficiency, or sepsis-meningitis may be heralded by neonatal seizures.
Prognostic Significance
Neonatal seizures are a powerful prognostic indicator of mortality and neurologic morbidity. The summary report from Bergman and associates (
2) of 1667 patients noted an overall mortality of 24.7% before 1969 and 18% after 1970. Volpe (
14) cited a mortality rate of 40% before 1969 and 20% after 1969. According to Lombroso (
15), mortality decreased modestly from about 20% previously to 16% in the early 1980s. These improvements probably reflect better obstetrical management and modern neonatal intensive care. All of these studies relied on seizure diagnosis by clinical criteria and did not require EEG confirmation.
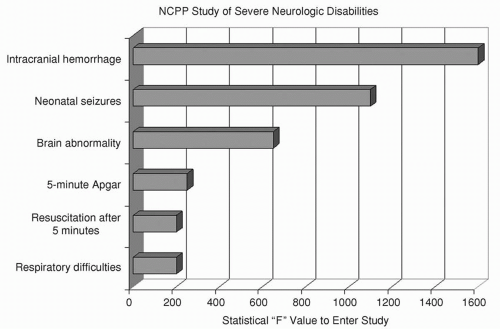
Survivors of neonatal seizures face an exceptionally high risk for cerebral palsy, often with mental retardation and chronic postnatal epilepsy. The National Collaborative Perinatal Population (NCPP) study (
16,
17) examined numerous clinical perinatal factors for their association with severe mental retardation, cerebral palsy, and microcephaly (
Fig. 32.3). The clinical diagnosis of “neonatal seizures” was independently and significantly associated with these adverse outcomes and eclipsed only by “intracranial hemorrhage” in forecasting them. Neurologic functioning may even be impaired in those who appear “normal” after neonatal seizures (
18).
Contemporary studies of the prognosis after neonatal seizures have emphasized the inclusion of infants whose seizure type was confirmed by EEG monitoring. Outcome has been assessed in terms of survival, neurologic disability, developmental delay, and postnatal epilepsy. Ortibus and colleagues (
19) reported that 28% died; 22% of survivors were neurologically normal at an average of 17 months of age; 14% had mild abnormalities; and 36% were severely abnormal. Six years later, Brunquell and colleagues (
20) put the mortality rate at 30%. Neurologic examinations showed abnormalities in 59% of survivors; 40% were mentally retarded; 43% had cerebral palsy; and 21% had postnatal epilepsy when followed up for a mean of 3.5 years.
Preliminary results of the Neonatal Seizures Clinical Research Centers from 1992 to 1997 have been reported (
21). Of the 207 full-term infants with video-electroencephalography-confirmed
seizures who were prospectively enrolled, 28% died. Two-year follow-up data were available for 122 patients, or 86% of the survivors. Abnormal neurologic findings were noted in 42%. A Mental Developmental Index (MDI) score below 80 was present in 55%, a Psychomotor Developmental Index (PDI) score less than 80 in 50%, and chronic postnatal epilepsy in 26%.
Whether seizures themselves adversely affect the developing brain is difficult to determine from clinical studies. Seizure
burden may appear to influence outcome because some infants who experience brief, infrequent seizures may have relatively good long-term outcomes, whereas those with prolonged seizures often do not fare as well. However, easily controlled or self-limited seizures may be the result of transient, successfully treated, or benign CNS disorders of neonates, while medically refractory neonatal seizures may stem from more sustained, less treatable, or more severe brain disorders. Legido and associates (
22) studied 40 neonates with electrographic seizures detected on randomly timed routine electroencephalogram examinations, and monitored them for cerebral palsy, mental retardation, and epilepsy. Overall neurologic outcome was more favorable in those with two or fewer seizures per hour than in those with more than that number. In the subgroup with seizures caused by asphyxia, cerebral palsy was more frequent when more than five seizures occurred per hour. However, these results might equally reflect more severe underlying injuries that triggered both the additional short-term seizures and greater morbidity on longterm follow-up. Attempting a balanced approach, McBride and coworkers (
23) followed up 68 high-risk neonates with birth asphyxia, meningitis, and other stressors linked to neonatal seizures. All infants underwent long-term EEG monitoring. Forty developed electrographic seizures, and 28 did not. By logistic regression, electrographic neonatal seizures were significantly correlated with death and cerebral palsy. Other investigators (
24), using proton magnetic resonance spectroscopy (
1H-MRS) found an association of seizure severity with impaired cerebral metabolism measured by lactate/choline and compromised neuronal integrity measured by
N-acetylaspartate/choline, and suggested this as evidence of brain injury not limited to structural damage detected by magnetic resonance imaging (MRI).
Neonatal Seizures May Be Inherently Harmful
Neonatal seizures may be intrinsically harmful to the brain (
25). Most seizures were long assumed to be the innocuous, albeit conspicuous, result of an acute injury, and the subsequent long-term neurodevelopmental abnormalities, the result of their underlying causes, not the seizures themselves. Basic animal research into how extensively seizure activity may affect the developing brain has not resolved the controversy (
26,
27,
28,
29,
30). Immature animals are more resistant than older animals to some seizure-induced injury (
31). The immature brain may be resistant to acute seizure-induced cell loss (
28); however, functional abnormalities such as impairment of visual-spatial memory and reduced seizure threshold (
32) occur after seizures, and seizures induce changes in brain development, including altered neurogenesis (
33), synaptogenesis, synaptic pruning, neuronal migration, and the sequential expression of genes including neurotransmitter receptors and transporters (
34,
35).
Research by Holmes and colleagues (
36) provides a newborn animal model for investigating whether recurrent seizures, induced by proconvulsant drugs that do not otherwise injure the brain, leave long-term undesirable effects on brain structure, learning, and susceptibility to spontaneous seizures. Starting on the first day of life, a series of about 25 seizures was induced by the administration of fluorothyl to neonatal rats. Behavioral testing in these animals as adults showed impaired spatial learning and memory, decreased activity levels, significantly lower threshold to pentylenetetrazol-induced seizures, and sprouting of CA3 mossy fibers, relative to controls that did not have neonatal seizures.
Neonatal seizures induce persistent changes in the inherent electrophysiologic properties of CA1 hippocampal cells in the rat (
37). Although the resting membrane potentials in CA1 pyramidal neurons did not differ in controls and neonatal-seizure animals, reductions were noted in CA1 spike frequency adaptation and afterpolarization potentials after a spike train.
Seizures also accelerate neuronal death in the neonatal rat hippocampus in the setting of hypoxia (
38). In the presence of hypoxia, the brain usually saves energy by blocking synaptic activity and electrically silencing the neuron; this effectively “turns off” the electroencephalogram. Seizures aggravate the hypoxic state by accelerating rapid anoxic depolarizations in the intact rat hippocampus. In effect, the generation of seizures “breaks the law of neuronal silence.”
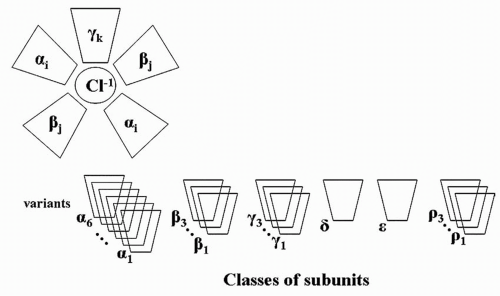
Finally, neonatal seizures in rats alter the subsequent composition of the GABA
A receptor. GABA
A is a pentamer in which five subunits assemble into a receptor structure (
Fig. 32.4). Six subunit classes may comprise the pentamer: six variants of alpha, three of beta, three of gamma, one of delta, one of epsilon, and three of rho. The specific composition of an individual GABA
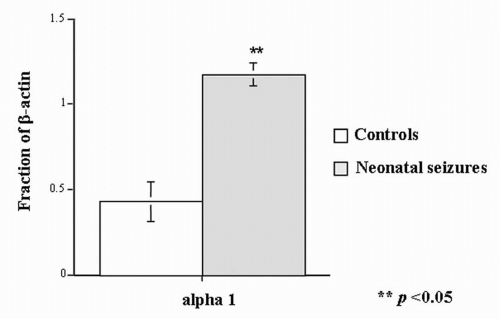
A receptor depends on developmental age. In the studies of Zhang and associates (
12,
39), rats with neonatal seizures had a substantially higher proportion of beta-actin in the α
1 GABA
A subunit component than did control animals (
Fig. 32.5) (
40).