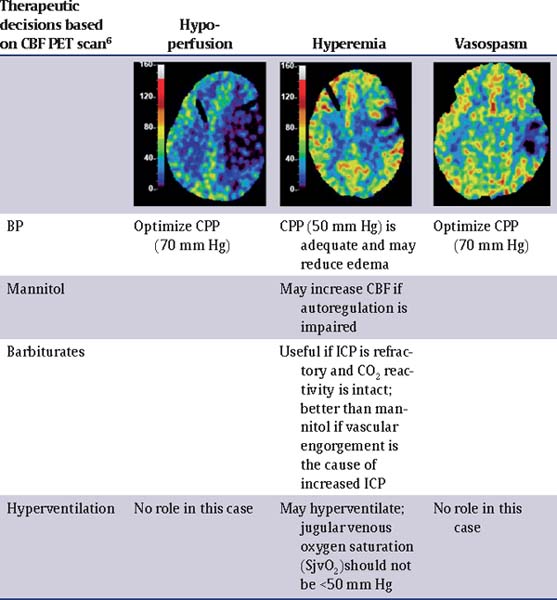
57 What are the amino acids commonly implicated in severe traumatic brain injury (TBI)? Glutamate and aspartate. Overactivation of N-methyl-D-aspartate (NMDA), 4-propionic acid, and kainic receptors causes influx of calcium (intracellular hypercalcemia plays a role in neurodegeneration).1 What is the role of oxygen free radicals? Excessive levels of highly reactive oxygen free radicals cause • lipid peroxidation of cell membranes and • oxidation of intracellular proteins and nucleic acids. Mannitol may act as a free radical scavenger. What is the early immune response to TBI? Suppression of the proliferative ability and concentration of T cells, IgG/M, and interleukin-2 (IL-2) responsive cells has been found immediately following trauma (these findings correlate with a higher number of infections during the first 4 days).2 What is the role of potassium (K) in the pathophysiology of TBI? Increased levels of extracellular K • disrupt the Na/K-ATPase cell membrane regulatory mechanism, leading to cell swelling (astrocyte swelling); and • are related to widespread neuronal depolarization and stimulate increased oxygen uptake in glial cells, depriving adjacent neurons of oxygen. What is the role of magnesium (Mg) in the pathophysiology of TBI? Decreased levels of extracellular Mg impair normal glycolysis, cellular respiration, and biosyntheses of DNA, RNA, and protein. Name the five most common metabolic responses to trauma. • Metabolic alkalosis • Increase water reabsorption • Increase Na+ reabsorption • Increased rate of lipolysis • Hyperglycemia3 What is the role of nitric oxide (NO) and nitric oxide synthase (NOS) in TBI? There is a triphasic (high-low-high) change in concentrations of NO in the brain after severe TBI. 1. The initial peak is due to the activity of endothelial NOS and neuronal NOS. 3-bromo-7-nitroindazole (7-NI) inhibits this immediate peak of NO and is effective in improving neurological outcome. 2. The subsequent episode of low NO is associated with low cerebral blood flow. Administration of L-arginine at this early time improves CBF and outcome. 3. The second peak in NO is due to activation of inducible NOS (iNOS). Inhibition of iNOS is neuroprotective.4 What is cerebral perfusion pressure (CPP)? CPP = Mean arterial pressure (MAP) – Intracranial pressure (ICP)5,6 What is cerebral autoregulation? It is the capacity to maintain blood flow at a relatively constant level during changes in blood pressure (BP) and is usually observed between a MAP of approximately 50 and 150 mm Hg. How does pressure autoregulation work? A decrease in CPP results in vasodilation and allows CBF to remain unchanged. This vasodilation can result in increased ICP, which further perpetuates the decrease in CPP (vasodilatory cascade); likewise, an increase in CPP results in vasoconstriction and may reduce ICP. What conditions can alter the cerebral metabolic rate of oxygen CMRO2? Head injury, anesthesia, and hypothermia decrease CMRO2. Fever and seizures increase CMRO2.7,8 How does the cerebral metabolic rate of glucose (CMRG) vary in head injury? CMRG may be elevated regionally and even globally when CMRO2 is reduced. What does this hyperglycolysis state indicate? Mitochondrial damage, inability to metabolize oxygen normally, and glucose depletion. This alteration can be another cause of secondary ischemic injury. What is the normal cerebral blood flow (CBF)? Normal CBF in humans averages 50 mL/100 g brain tissue per minute. What are the three different groups of patients with severe TBI based on CBF? • Low CBF (<33 mL/100 g/min); these patients are more prone to develop cerebral ischemia. • Relative hyperemia (33–55 mL/100 g/min) • Absolute hyperemia (>55 mL/100 g/min)5 Is it ever appropriate to place an ICP monitor in a patient with a normal CT? Yes,9–11 based on the Guidelines for the Management of Severe TBI 2007. Class II evidence: ICP should be monitored in all salvageable patients with a severe TBI (GCS score of 3 to 8 after resuscitation) and an abnormal CT (see below). Class III evidence: ICP should be monitored in patients with severe TBI with a normal CT if two or more of the following factors are noted at admission: age over 40 years, unilateral or bilateral motor posturing, or systolic blood pressure <90 mm Hg. What is considered an abnormal CT? An abnormal CT is one that reveals hematomas, contusions, swelling, herniation, or compressed basal cisterns.12 Is the Camino bolt the “gold standard” for ICP monitoring? No,9–11 based on the Guidelines for the Management of Severe TBI 2007. A ventricular catheter connected to an external strain gauge is the most accurate, cost-effective, and reliable method of monitoring ICP. It can be recalibrated in situ. Parenchymal ICP monitors cannot be recalibrated during monitoring. Parenchymal ICP monitors, using micro strain pressure transducers, have negligible drift. Subarachnoid, subdural, and epidural monitors are less accurate. What are some of the complications of ventriculostomy catheter placement? Infection incidence of 5 to 14% (a nonlinear increase of risk in the first 10 to 12 days, after which the rate diminished) Other complications (overall incidence of 1.4%) include hemorrhage as well as catheter malfunction, obstruction, and malposition. What is the only factor that will significantly reduce the risk of infection? Appropriate sterile technique What are the factors that are not associated with infection? • Insertion of catheter in NICU vs. OR • Previous catheter • Drainage of CSF • Use of steroids Does an antibiotic-coated catheter decrease the risk of infection? Yes, antibiotic-coated catheters reduce the risk of infection (ranges of infection rates are from 9.4 to 17% for nonimpregnated catheters to 1.3 to 2.4% for impregnated catheters13,14). Is it good practice to routinely exchange EVD catheters to reduce infection risk? No,15 based on the Guidelines for the Management of Severe TBI 2007 regarding infection prophylaxis. Class III evidence: Routine ventricular catheter exchange or prophylactic antibiotic use for ventricular catheter placement is NOT recommended to reduce infection. What first-tier interventions can be done to decrease ICP? • Head elevation to 30 degrees and in neutral position • Airway and ventilation control • Sedation and analgesia • Treatment of anemia • Control of fever • Control of hypertension • Prevention of seizures16 Will ICP always be low after a large intracranial hematoma evacuation? No,3 this is a misconception. Intracranial hypertension (IC-HTN) will occur in 50 to 70% of patients with evacuated intracranial hematoma. What are some of the factors that contribute to increased ICP? • Traumatically induced masses • Cerebral edema (after evacuation of mass lesions, this is the primary cause) • Hyperemia owing to vasomotor paralysis • Hypoventilation that leads to hypercarbia • Hydrocephalus • Increased intrathoracic and intraabdominal pressure (from mechanical ventilation, posturing, agitation, Valsalva maneuvers) Is a CPP of 70 mm Hg an appropriate goal? Based on the Guidelines for the Management of Severe TBI 20072: Class II evidence: Aggressive attempts to maintain CPP above 70 mm Hg with fluids and pressors should be avoided because of the risk of adult respiratory distress syndrome (ARDS). Class III evidence: CPP <50 mm Hg should be avoided. The CPP target value lies within the range of 50 to 70 mm Hg. Patients with intact pressure autoregulation tolerate higher CPP values. Is it OK to have SBP <90 mm Hg, as long as PaO2 is >90? This should usually be avoided,17 based on the Guidelines for the Management of Severe TBI 2007. Class II evidence: Blood pressure should be monitored and hypotension (SBP <90 mm Hg) avoided. Class III evidence: Oxygenation should be monitored and hypoxia (PaO2 <60 mm Hg or O2 saturation <90%) avoided. What is the antihypertensive agent of choice to treat hypertension in TBI? Sympathomimetic-blocking • beta-blocking drugs (labetalol, esmolol) • central-acting α-receptor agonists (clonidine) Why are sympathomimetic-blocking antihypertensives the agents of choice to treat hypertension in TBI? Because they reduce blood pressure without affecting the ICP Are there selected TBI patients in whom steroids are indicated? Class I evidence: Steroids are not recommended for improving outcome or reducing ICP. In patients with moderate or severe TBI, high-dose methylpred nisolone is associated with increased mortality and is contraindicated (CRASH [Corticosteroid randomisation after significant head injury] trial18). What are the measures used to treat medically refractory intracranial hypertension (second tier)? In this order: • Heavy sedation and paralytics • Hyperosmolar therapy • Hyperventilation • Barbituric coma • Hypothermia (this last one is controversial) Has the use of propofol supplanted pentobarbital in the management of the TBI patient? Based on the Guidelines for the Management of Severe TBI 200716,19: Class II evidence: Prophylactic administration of agents to induce burst suppression EEG is not recommended. High-dose barbiturate administration is recommended to control elevated ICP refractory to maximum standard medical and surgical treatment. Hemodynamic stability is essential before and during barbiturate therapy. Propofol is recommended for the control of ICP, but not for improvement in mortality or 6-month outcome. High-dose propofol can produce significant morbidity. Describe the propofol infusion syndrome. Common clinical features include hyperkalemia, hepatomegaly, lipemia, metabolic acidosis, myocardial failure, rhabdomyolysis, and renal failure resulting in death19 What is the dose in which the propofol infusion syndrome can occur? Extreme caution when using doses >5 mg/kg/h or >8 μg/kg/min or when usage exceeds 48 hours What other alternatives can be used for sedation? • Morphine sulfate, although tachyphylaxis is extremely common • Fentanyl and sufentanil have become increasingly popular because of their brief duration of action. However, these agents have been shown to cause a mild but definite elevation in ICP. Which anesthetic agent decreases CBF and CRMO2 and suppresses adrenocortical response? Etomidate19 Which anesthetic agent decreases CBF and CRMO2 and produces cardiovascular depression? Thiopental19 Which anesthetic agent induces seizures discharges? Enflurane19 What are the major complications of neuromuscular blockade? • Myopathy (increased when the neuromuscular blocking agent is combined with β2-agonists, corticosteroids or antibiotics [aminoglycosides]) • Polyneuropathy • Prolonged neuromuscular blockage Is hypertonic saline (HTS) equivalent to mannitol for treatment of elevated ICP? For the most part, yes,20,21 based on the Guidelines for the Management of Severe TBI 2007. Class II evidence: Mannitol is effective for control of raised ICP at doses of 0.25 g/kg. Arterial hypotension (SBP <90 mm Hg) should be avoided. Class III evidence: Restrict mannitol use prior to ICP monitoring in patients with signs of transtentorial herniation or progressive neurological deterioration not attributable to extracranial causes. In a recent study on 47 TBI patients,21 mannitol was shown to be as effective as HTS in decreasing ICP, although both failed to improved cerebral metabolism. HTS also demonstrated a stronger effect on cerebral perfusion in the presence of cerebral ischemia. What are the mechanisms by which mannitol exerts its beneficial effect? Rheological and osmotic effects20 What are the rheological effects of mannitol? • Immediately plasma-expanding • Reduction of hematocrit • Increases deformability of erythrocytes • Reduction of blood viscosity • Increases CBF and oxygenation What is important to know about the osmotic effects of mannitol? Mannitol takes from 15 to 30 minute to be effective and will increase serum tonicity. Stop mannitol when osmolarity reaches 320 mOsm to prevent hypovolemia, hyperosmolarity, and renal failure. Why is it better to administer mannitol in boluses that as an infusion? To prevent rebound edema20 What is the dose of mannitol typically given for reduction of ICP? • A bolus of 0.25 to 1 g/kg • 1 g/kg should be given when urgent reduction of ICP is needed. • Higher doses (1.4 g/kg) may give significantly better results in extreme critical situations. What are the mechanisms by which hypertonic saline exerts its effect? • Osmotic mobilization of water across the blood–brain barrier (BBB) • Dehydration of endothelial cells and erythrocytes, which increases the diameter of the vessels and deformability of erythrocyte leading to an increase in CBF • Reduces leukocyte adhesion in the traumatized brain20 In which scenario does hypertonic saline have an advantage over mannitol? In hypotensive and hypovolemic patients (a very common scenario in TBI) Is hyperventilation ever indicated in TBI? Based on the Guidelines for the Management of Severe TBI 200722: Class II evidence: Prophylactic hyperventilation (PaCO2 of 25 mm Hg or less) is not recommended. Class III evidence: Hyperventilation is recommended as a temporizing measure for the reduction of elevated ICP. Hyperventilation should be avoided during the first 24 hours after injury when CSF is often critically reduced. If hyperventilation is used, jugular venous oxygen saturation (SjvO2) or brain oxygen tension (PbrO2) measurements are recommended to monitor oxygen delivery. Explain the rebound effect of hyperventilation. The vasoconstrictive effect on cerebral arterioles lasts 11 to 20 hours because the pH of the CSF equilibrates to the new PaCO2, and then cerebral arterioles re-dilate (possibly to a larger caliber). When hypocarbia is induced and maintained for hours, it should be reversed slowly (over days) to minimize this rebound hyperemia.22 • Loading dose 10 mg/kg over 30 minute; 5 mg/kg every hour × 3 doses • Maintenance 1 mg/kg/h • Titrate to serum levels of 30 to 50 μg/mL or burst suppression pattern on EEG23 What is the mechanism of action of barbiturates to lower ICP? Unclear, but likely reduces CBF and CMRO2; its action is closely tied to the retention of CO2
Neurocritical Care
57.1 Molecular Concepts
57.2 Cerebral Metabolism
57.3 ICP Monitoring
57.4 ICP Management
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






