Virus (Genotype)
Year
Location
Age of patient
Reference
Mokola (3)a
1968
Nigeria
3.5
Familusi and Moore (1972)
Mokola (3)
1971
Nigeria
6
Familusi et al. (1972)
Duvenhage (4)
1970
South Africa
31
Meredith et al. (1971)
Duvenhage (4)
2006
South Africa
77
Paweska et al. (2006)
Duvenhage (4)
2007
Kenya
34
van Thiel et al. (2009)
European Bat Lyssavirus 1 (5)
1985
Russia
11
Selimov et al. (1989)
European Bat Lyssavirus 1 (5)
2002
Ukraine
34
Botvinkin et al. (2005)
European Bat Lyssavirus 2 (6)
1985
Finland
30
Roine et al. (1988)
European Bat Lyssavirus 2 (6)
2002
Scotland
55
Australian Bat Lyssavirus (7)
1996
Australia
39
Samaratunga et al. (1998)
Australian Bat Lyssavirus (7)
1998
Australia
37
Hanna et al. (2000)
Australian Bat Lyssavirus (7)
2013
Australia
8
Anonymous (2013)
Irkut (pending)
2007
Russia
20
Leonova et al. (2009)
2 Epidemiology
Globally about 55,000–75,000 humans die from rabies every year. Around half of the victims of rabies are children. Hence, rabies remains a very significant public health problem and is an important neglected disease. Surveillance for human rabies is poor, particularly in countries where the disease is prevalent. Laboratory confirmation diagnosis is infrequent and clinical diagnosis is inaccurate. Worldwide about 99 % of human cases of rabies are transmitted from dogs. Canine rabies continues to be a large problem in Asia and Africa. About three billion humans live in regions with endemic dog rabies, which puts a large proportion of the human population at risk of developing rabies (Knobel et al. 2007). In these regions there is dog-to-dog transmission of rabies virus. For a variety of economic, political, religious, and cultural reasons, canine rabies has not been controlled in many countries, although the methods of how to do this are very well developed and a “blueprint” for rabies control has recently been published (Lembo 2012) and is available on the Internet (http://www.rabiesblueprint.com/). Canine rabies has been well controlled in most of Latin America over the past few decades with dog control measures, particularly employing mass vaccination of dogs. There are about 30,000 human deaths from rabies each year in India. China recently had a resurgence of rabies, which is also related to endemic canine rabies, with a peak of over 3,300 human deaths in 2007 (Hu et al. 2009). Thailand markedly reduced the death rate due to human rabies from hundreds of cases to about ten cases per year by developing a very effective post-exposure rabies prophylaxis program without controlling dog rabies in the country. Outbreaks of rabies continue to occur in focal areas. For example, in 2008 an outbreak of human rabies deaths began on the island of Bali, Indonesia.
Rabies is also endemic in wildlife and typically a rabies virus variant becomes adapted to a particular species and can be readily transmitted within that species. However, when this variant is transmitted to another species, the new host is fully susceptible but cannot usually transmit the virus further and becomes a “dead-end” host. Rabies virus variants can be characterized from clinical specimens (e.g., saliva or skin biopsies) by using reverse transcription polymerase chain reaction (RT-PCR) amplification and sequencing or by characterization using monoclonal antibodies, which allows identification of the species of the vector that the variant is associated with and is also useful in identifying or confirming the animal source of human cases.
Bats are the most important wildlife vector. The majority of cases of human rabies occurring in the United States and Canada are caused by bat rabies virus variants. Many human cases of rabies due to these variants have no history of bat contact (38 %) or have either a house exposure with no direct contact (10 %) or direct contact with no recognized bite (18 %); only 38 % of cases are associated with a history of a bite or scratch (Table 2). The rabies virus variant associated with silver-haired bats (Lasionycteris noctivagans) and tricolored bats (previously called eastern pipistrelle bats) (Perimyotis subflavus) is responsible for most human rabies cases in North America. These bats are not normally found in homes, in contrast to little brown bats (Myotis lucifugus) and big brown bats (Eptesicus fucus), which are often found to have rabies, but variants associated with these bats are only infrequently responsible for human rabies cases. The second most common rabies virus variant causing human rabies in the United States is found in Brazilian (Mexican) free-tail bats (Tadarida braziliensis). Vampire bats also transmit rabies virus to humans and cattle in Latin America. In recent years there have been outbreaks in Brazil and Peru in indigenous populations in the Amazonian region (Schneider et al. 2009). Other genotypes of lyssaviruses (rabies-related viruses) are endemic in bats in Europe, Africa, Asia, and Australia and very rarely cause clinical illness identical to rabies (Table 1). Many human cases have likely gone unrecognized, particularly in Africa, because of the presence of endemic canine rabies.
Table 2
Indigenously acquired cases of human rabies from bats in the United States and Canada, 1950–2010a
Type of case | Number of cases |
|---|---|
Bite or scratch | 23 (37.7 %) |
Direct contact with no recognized bite | 11 (18.0 %) |
House exposure, but no direct contact | 6 (9.8 %) |
No history of bat contact | 21 (34.4 %) |
Total | 61 |
Terrestrial wildlife rabies vectors include raccoons, skunks, foxes, and coyotes (Fig. 1). Raccoon rabies was first identified in Florida in the 1940s and has gradually spread north and currently has a distribution involving the entire eastern coast of the United States. Raccoon rabies has had multiple incursions into Canada from the United States beginning in 1999 (Ontario), but has been very effectively controlled and eradicated by Canadian wildlife control operations. In 2012 there were 1,953 laboratory-confirmed cases of raccoon rabies by passive surveillance in the United States (Dyer et al. 2013). Only two cases of human rabies due to the raccoon rabies virus variant have been recognized to date (Silverstein et al. 2003; Vora et al. 2013). Skunk rabies occurs mainly in the midwestern United States, California, and in the prairie provinces of Canada. Human cases have not occurred from skunk virus variants in recent decades. Fox rabies has been well controlled in Europe, Ontario, and Texas with oral immunization methods, and is not currently an important problem (Rosatte 2013). Coyote rabies was also controlled in Texas with oral immunization. There are a variety of other terrestrial vectors of rabies, including mongooses, wolves, and jackals. Lagomorphs (e.g., rabbits and hares) and rodents are not considered rabies vectors and exposures from small rodents, including mice, rats, squirrels, chipmunks, gerbils, hamsters, and guinea pigs, have not been known to transmit infection to humans (Manning et al. 2008). Woodchucks are responsible for the majority of rabies in rodents that is reported in the United States (Childs et al. 1997). However, all mammals are considered potentially susceptible to rabies. Opossums are considered relatively resistant to rabies (Baer et al. 1990).
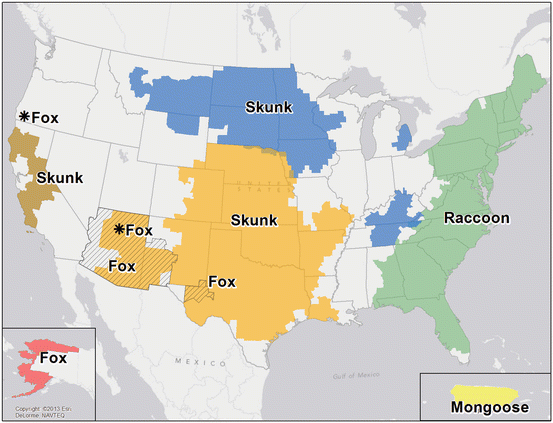

Fig. 1
Distribution of the major rabies virus variants among wild terrestrial reservoirs in the United States and Puerto Rico, 2008–2012. *Potential host shift event (Reproduced from JL Dyer et al., J Am Vet Med Assoc 243:805–815, 2013, Centers for Disease Control and Prevention)
3 Clinical Features of Human Disease
The vast majority of human rabies cases are associated with rabies virus transmission via an animal bite. A scratch contaminated with saliva or salivary contamination of an open wound may allow transmission, but this is much less common. Aerosol transmission in laboratory accidents and in caves containing millions of bats is a rare route of transmission. Finally, transmission via transplantation of tissues, including cornea (eight cases) and a vascular conduit (one case), or organs (seven cases) is well documented (Burton et al. 2005; Srinivasan et al. 2005; Maier et al. 2010; Jackson 2013; Vora et al. 2013), but rare.
In comparison to many other infectious diseases, the incubation period is relatively long in rabies, typically lasting 20–90 days. However, there are well documented cases with incubation periods of up to 6 years (Smith et al. 1991). Current evidence indicates that during the vast majority of the incubation period the virus remains close to the site of entry (inoculation). The nicotinic acetylcholine receptor, which is present on the post-synaptic membrane of the neuromuscular junction, is a rabies virus receptor and may serve to concentrate the virus and facilitate invasion into the peripheral nervous system (Lentz et al. 1982). Rabies virus spreads within axons by retrograde fast axonal transport and invades either the spinal cord or brainstem, depending on the site of entry. Once rabies virus enters the CNS the virus spreads throughout the CNS along neuroanatomical connections intraaxonally by fast axonal transport. Subsequently, there is centrifugal viral spread from the CNS to multiple organs along sensory and autonomic nerves. This is particularly important in rabies vectors for spread to the salivary glands, where rabies virus is secreted in high titer in the saliva, which is important for transmission to new hosts. The virus also spreads to the skin around hair follicles, which forms the basis of the skin biopsy as a diagnostic test for rabies. There is spread to all organs via nerves, including the heart, gastrointestinal tract, and adrenal medulla, and, hence, organs or tissues from rabies patients cannot be transplanted without a high risk of viral transmission. A minority of rabies patients develop myocarditis related to the cardiac involvement (Cheetham et al. 1970; Raman et al. 1988; Ross and Armentrout 1962).
Prodromal symptoms of rabies, which last for up to 10 days, are usually non-specific and include fever, chills, malaise, fatigue, insomnia, anorexia, headache, anxiety, and irritability. The earliest neurological symptoms, which are highly suggestive of rabies, are paresthesias, pain, and pruritus at the site of the exposure. These symptoms usually occur close to the site of viral entry, and the bite wound may have completely healed by this point. They are due to infection and associated inflammatory changes in local dorsal root ganglia.
There are two clinical forms of rabies: an encephalitic (furious) form in about 80 % of patients and a paralytic (dumb) form in about 20 %. The main burden of infection in encephalitic rabies is likely in the brain, whereas it likely mainly involves the spinal cord, spinal nerve roots, and nerve plexuses in paralytic rabies. Fever occurs in both clinical forms. In encephalitic rabies, patients characteristically have episodes of generalized arousal or hyperexcitability, which are separated by lucid periods (Warrell 1976). The patients may demonstrate aggressive behavior, confusion, and hallucinations. Signs of autonomic dysfunction are common and include hypersalivation, piloerection (gooseflesh), sweating, priapism, and cardiac arrhythymias. About half of patients with encephalitic rabies develop hydrophobia, which is the most characteristic manifestation of rabies. Prior to developing hydrophobia, patients may experience pain in the throat or have difficulty swallowing. On attempts to swallow, patients with hydrophobia experience contractions of the diaphragm and other inspiratory muscles that usually last for about 5–15 s. Subsequently, the sight, sound, or even mention of water (or of any liquids) may trigger the spasms. A draft of air on the skin may have the same effect, and this is called aerophobia. Clinically the disease progresses with the subsequent development of paralysis and coma and virtually always results in a fatal outcome.
In paralytic rabies flaccid muscle weakness of the lower motor neuron variety develops early in the course of the disease and typically begins in the bitten extremity and then spreads to involve the other extremities and also involves the facial muscles. Sphincter involvement, pain, and sensory disturbances also occur. Bulbar and respiratory muscles eventually develop weakness in paralytic rabies, but the development of hydrophobia is unusual. Patients with paralytic rabies also progress to coma and death, and they typically survive longer than patients with encephalitic rabies.
Medical complications occur very frequently in rabies patients treated aggressively in critical care units. Cardiac and respiratory complications are particularly common. Cardiac problems, including sinus tachycardia, heart failure, hypotension, a variety of cardiac arrhythmias, and cardiac arrest (Hattwick 1974; Warrell et al. 1976), occur frequently in rabies. The cardiac manifestations probably reflect infection involving the autonomic nervous system (e.g., cardiac ganglia) and myocardium (Jackson et al. 1999). Respiratory complications include hyperventilation, hypoxemia, respiratory depression with apnea, atelectasis, and aspiration pneumonia (Hattwick 1974). Hypothalamic involvement in rabies may produce either hyperthermia or hypothermia. Endocrine complications include inappropriate secretion of antidiuretic hormone and diabetes insipidus (Bhatt et al. 1974; Hattwick 1974). Multiple organ failure commonly develops in patients who are treated aggressively in critical care units.
4 Diagnostic Investigations
Routine blood tests are usually normal in rabies. Computed tomography (CT) imaging is usually normal. Magnetic resonance imaging may show non-specific signal abnormalities in brain, spinal cord, nerve roots and/or plexuses (Laothamatas et al. 2011). Cerebrospinal fluid (CSF) analysis usually shows a mild mononuclear cell CSF pleocytosis with a cell count less than 100 cells per μL, although pleocytosis may be absent. In a previously unvaccinated patient neutralizing serum anti-rabies virus antibodies may develop, but this may not occur until illness is present for days to weeks. The presence of neutralizing anti-rabies virus antibodies in CSF is thought to be diagnostic of rabies, whereas these CSF antibodies are absent in patients vaccinated against rabies.
A laboratory diagnosis of rabies depends on the detection of rabies virus antigen or RNA in a body fluid or a tissue. Rabies virus can be isolated with viral cultures from CNS tissues, but only rarely from other clinical specimens such as saliva, CSF, or urine. Tissue sections from a full thickness skin biopsy (usually 5–6 mm in diameter) containing hair follicles (minimum of 10), which can be obtained from the posterior region of the neck at the hairline, should be evaluated for the presence of rabies virus antigen using the direct fluorescent antibody technique (Warrell et al. 1988). Corneal impression smears have relatively low sensitivity for the detection of rabies virus antigen (Warrell et al. 1988) and are now rarely used. The important recent advance in the laboratory diagnosis of rabies is an assay for the presence of rabies virus RNA in saliva using reverse transcription polymerase chain reaction (RT–PCR) amplification. RT–PCR can also be used on skin biopsy specimens (Dacheux et al. 2008) and for CSF, but the sensitivity in CSF is much lower. A negative test for the detection of rabies virus antigen or RNA (except on brain tissues) never excludes rabies. Repeat testing may be necessary to confirm a diagnosis of rabies. Biopsy specimens of brain tissues or post-mortem brain tissues can be evaluated for the detection of rabies virus antigen and for rabies virus isolation using culture techniques. Pathologically, inflammatory changes are observed in the nervous system and characteristic eosinophilic inclusion bodies called Negri bodies (Fig. 2) are seen in the cytoplasm of neurons.
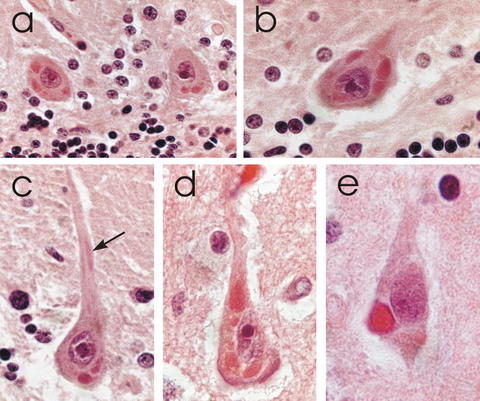

Fig. 2
Hematoxylin and eosin stained sections showing Negri bodies in the perikarya of (a–c) cerebellar Purkinje cells and (d, e) pyramidal neurons in the cerebral cortex of human rabies cases. The arrow in (c) indicates a Negri body in an apical dendrite. (Magnifications: A, ×280, B, ×445, C, ×535, D, ×710, E, ×840) (Adapted with permission from Rossiter JP and Jackson AC: Pathology, in Rabies: scientific basis of the disease and its management, Third Edition, edited by AC Jackson, 2013, Elsevier Academic Press, Oxford, UK, pp 351–386; Copyright Elsevier)
5 Human Rabies Due to Other Lyssaviruses
Rabies virus (genotype 1) is in the genus Lyssavirus that includes 10 other genotypes. Five of these 10 genotypes have been recognized to very rarely cause human disease in which the clinical and laboratory features are indistinguishable from rabies (Table 1). Cases due to other lyssaviruses have occurred in Africa (5, but one doubtful) and Europe (4) and less commonly in Australia (3) and Asia (1). Interestingly, no cases have been reported from the Americas.
6 Prognosis in Human Rabies
Rabies is virtually always fatal. Most surviving cases have received rabies vaccine prior to the onset of their illness (Table 3). One case did not receive rabies vaccine, but had the presence of neutralizing anti-rabies virus antibodies at the time of clinical presentation (Willoughby et al. 2005). A 17-year-old female from Texas (Holzmann-Pazgal et al. 2010) and an 8-year old female from California (Wiedeman et al. 2012) had atypical clinical courses, with the former patient not even requiring critical care, and both survived their illnesses. Rabies virus antibodies were detected in these two patients, but they had only a low titer or absence of rabies virus neutralizing antibodies in sera and no detectable neutralizing anti-rabies virus antibodies in CSF and all other diagnostic tests for rabies were negative. The absence or minimal development of rabies virus neutralizing antibodies indicates that it is unlikely that either of these patients actually developed rabies and recovered. These two patients should not be considered rabies survivors.
7 Management of Human Rabies
All health care workers should initiate body substance precautions as soon a diagnosis of rabies is seriously considered and wear gowns, gloves, masks, and eye protection. They may also require post-exposure prophylaxis after high-risk contact with a patient with rabies.
Most survivors of rabies have received doses of rabies vaccine prior to the onset of their illnesses. An expert committee published a detailed document in 2003 on considerations for potential therapies for patients with rabies (Jackson et al. 2003). The best potential candidates for aggressive therapy were felt to be young and previously healthy patients with an early clinical diagnosis of rabies (Jackson et al. 2003). Therapies suggested for consideration include rabies vaccine, human rabies immune globulin, monoclonal antibodies (for the future), ribavirin, interferon-α, and ketamine. Previous animal work indicated that ketamine therapy might be useful (Lockhart et al. 1991). It was felt that combination therapies might be useful in situations in which specific therapies used individually had failed in the past; this approach has proved to be effective for both other infectious and non-infectious diseases,
In 2004 a 15-year-old female patient, who had bitten by a bat and not received rabies vaccine or other post-exposure therapy prior to the onset of clinical disease, survived rabies (Willoughby et al. 2005). After an incubation period of about a month she developed typical clinical features of rabies encephalitis. Five days after the onset of neurological symptoms she was transferred to a tertiary care hospital in Milwaukee, Wisconsin, and neutralizing anti-rabies virus antibodies were detected in both her sera and CSF. Nuchal skin biopsies were negative for rabies virus antigen. Rabies virus RNA was not detected in the skin biopsies or in saliva by RT – PCR, and viral isolation on saliva was negative. She was intubated, and put into a drug-induced coma, which included the non-competitive N-methyl-d-aspartate (NMDA) antagonist ketamine at 48 mg/kg/day as a continuous infusion and intravenous midazolam for 7 days. A burst-suppression pattern on her electroencephalogram was maintained with supplemental phenobarbital given as needed. Antiviral therapy included intravenous ribavirin and amantadine 200 mg per day administered enterally. She improved and was discharged from hospital with neurologic deficits and she subsequently demonstrated showed further neurologic improvement (Hu et al. 2007).
It is unknown if therapy with one or more specific agents played a significant role at all in her favorable outcome (Jackson 2005). Since that time there have been at least 26 cases in which the main components of this approach (the “Milwaukee Protocol”) have been used with fatal outcomes (Table 4), and there have no documented survivors. The induction of coma per se has no established benefit for the management of infectious diseases of the nervous system, and to date there is no evidence supporting this approach in rabies or other viral encephalitides. There is no real justification for therapeutic coma becoming a routine therapy for the management of rabies. Excitotoxicity is not supported as an important basic mechanism in the pathogenesis of rabies and further studies on ketamine therapy both in vitro and in vivo have indicated a lack of efficacy (Weli et al. 2006). It is very likely that the Milwaukee patient would have recovered if she had received only supportive therapy.
Table 3
Cases of human rabies with recoverya
Location | Year | Age of patient | Transmission | Immunization prior to onset | Outcome | Reference |
|---|---|---|---|---|---|---|
United States | 1970 | 6 | Bat bite | Duck embryo vaccine | Complete recovery | Hattwick et al. (1972) |
Argentina | 1972 | 45 | Dog bites | Suckling mouse brain vaccine | Mild sequelae | Porras et al. (1976) |
United States | 1977 | 32 | Laboratory (vaccine strain) | Pre-exposure vaccination | Sequelae | Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|





