Fig. 1
Coronal section of the brain showing several vesicular cysticerci
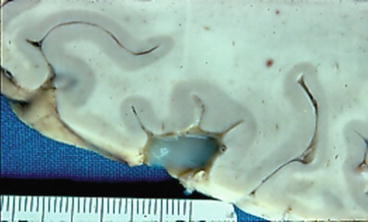
Fig. 2
Coronal section of the brain showing colloidal cysticerci
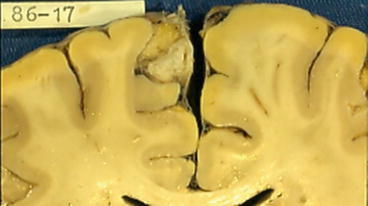
Fig. 3
Coronal section of the brain showing a calcified cysticercus
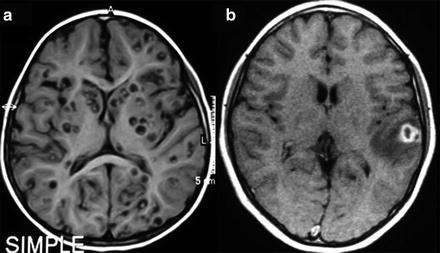
Fig. 4
MRI of parenchymal neurocysticercosis. (a) Viable cysts showing the scolex. (b) Colloidal cyst appearing as a ring-enhancing lesion with perilesional edema
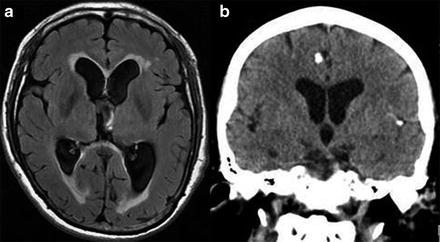
Fig. 5
Imaging findings in patients with neurocysticercosis. (a) MRI showing hydrocephalus with transependymal edema. (b) CT showing calcified cysticerci associated with ventriculomegalia
Genetic diversity of cysticerci collected in pork has been investigated. The comparison of parasites from different continents shows significant genetic differentiation, suggesting diverse evolutionary paths (Vega et al. 2003; Maravilla et al. 2003). On the other hand, when cysticerci of the nearest geographical regions or from different tissues of the same pig were studied, DNA diversity was within the range expected from a recombination process (Vega et al. 2003; Bobes et al. 2010).
The genome of T. solium is currently being sequenced in Mexico and only partial results have been published at the time of this review. The information generated will provide a powerful resource for the study of taeniasis/cysticercosis helping to address fundamental questions such as the molecular basis of host-parasite specificity and mechanisms of parasite pathogenesis, among others (Aguilar-Díaz et al. 2006). Genome size has been estimated to be between 251 and 270 Mb and only about 24 % of the sequences are homologous to mammalian genes, in particular humans. The sequences lacking homologues in humans are strong candidates for investigation of treatment, diagnosis and vaccination (Aguilar-Díaz et al. 2006).
4 Immunological Response
In their larval phase, cysticerci express a complex and diverse set of antigens. A few of them are specific of T. solium, but many others cross-react with those of other helminths. Some of them are present in all the phases of the parasites, while others are phase-specific. Most patients have detectable antibodies, generally of the IgG class, against parasites in serum and cerebrospinal fluid (CSF). They can also be detected in the saliva (Bueno et al. 2000), The four subclasses of IgG are increased in symptomatic NC patients compared to the asymptomatic patients. The relevance of antibodies to parasite damage has not been extensively studied. Although some observations indicate that antibodies can have a protective action, the demonstration of a net beneficial effect is still lacking.
Characteristics of the local inflammatory reaction associated with the presence of the parasite depend on the CNS compartment where the parasites are located. When located in the parenchyma or in the subarachnoid space, inflammation is confined around the parasite. In these cases, the CSF is in general normal and immunohistochemical studies of the inflammatory infiltrate around the cysticerci reveals the presence of specific IgM and plasma cells, natural killer (NK) lymphocytes, macrophages, granulocytes, and T cells. Intensity of the inflammatory reaction varies, based upon parasite evolutionary phase, being almost absent in the vesicular phase and intense in the colloidal one (Alvarez et al. 2002). When the parasites are located in the basal subarachnoid space or in the ventricular system, inflammation is mainly seen in the CSF with the presence of lymphocytic pleocytosis, mild elevation of proteins, and hypoglycorrhachia, with increased levels of all IgG subclasses as well as interleukin (IL) -6, -5, and -10 (Chavarría et al. 2005). This reaction can affect the leptomeninges with the formation of a dense exudate containing collagen fibers, lymphocytes, and multinucleated giant cells resulting in meningeal thickening as well as occlusion of Luschka and Magendie foramina that can lead to chronic hydrocephalus (Escobar 1983). In these cases, inflammation can cause damage in vascular and nervous structures remote from the parasites.
Systemic immunological changes are also observed. In the blood of patients with inflammatory parasites located in the subarachnoid space or in the ventricular system, a decreased proliferation of specific cells without production of cytokines, and an increase of the proportion of T regulatory cells, significantly correlated with the increase of these cells in CSF, have been observed (Chavarria et al. 2006; Adalid-Peralta et al. 2012). On the other hand, patients with asymptomatic calcified NC present a specific proliferative reaction, with production of cytokines predominantly of the Th2 type (IL-4, IL-5, IL-13, IL-12) and a higher level of specific IgG4, compared to persons exposed to parasites but non-infected (Chavarría et al. 2003).
Parasites have developed different protective mechanisms to evade the immune surveillance of the host. Particularly, antigen B, the most frequent antigen recognized by the serum of patients, can bind factor C1q from the complement system, a property that can prevent the potential toxicity of antibody-mediated parasite damage (Laclette et al. 1992). The presence of a great amount of immunoglobulin on the surface of the cysticercus can mask its presence in the immunological system (Flisser et al. 1986).
5 Clinical Manifestations
One of the most intriguing aspects of NC is that presumably a high percentage of the individuals harboring NC remain asymptomatic. Among the symptomatic group, clinical manifestations of NC are determined mainly by the evolutive phase and location of the parasite within the CNS, as well as by the intensity of the immunological response of the patient (Loureiro das Chagas et al. 2003; Patel et al. 2006). The combination of these factors, among others, explains the great heterogeneity and the absence of a specific clinical picture. A systematic review was conducted to estimate the clinical manifestation frequencies of symptomatic NC (Carabin et al. 2011). Among NC patients seen in neurology clinics, about 79 % had seizures/epilepsy, 38 % severe headaches, 16 % focal deficits, and 12 % signs of increased intracranial pressure. Several other symptoms were also reported in less than 10 % of the patients.
5.1 Parenchymal Neurocysticercosis
The most common clinical manifestation of parenchymal NC is epileptic seizure, which occurs in 60–90 % of cases. The symptomatology of altered mental state and psychiatric manifestations remains poorly described in the literature (Carabin et al. 2011). In two studies (Forlenza et al. 1997; Carpio et al. 2008) which provided definitions of clinical manifestations, depression was reported in about 53 % and 15 % of the patients, respectively. A recent study reports a spectrum of cognitive abnormalities, including dementia (Rodrigues et al. 2012).
5.1.1 Neurocysticercosis and Epilepsy
Seizures associated with NC may be categorized as either acute symptomatic (Carpio et al. 1998; Murthy and Yangala 1999) or as unprovoked, remote symptomatic (epilepsy, if recurrent). This differentiation is very important, due to its implications concerning treatment and prognosis. The classification of seizure types in patients with NC varied among studies (Cukiert et al. 1994). It seems that either generalized seizures or partial seizures with secondary generalization are most commonly reported, while complex partial seizures are less frequent (Carpio et al. 2013). Seizures may occur at any evolutionary phase of the parasite. In a recent prospective cohort study (Kelvin et al. 2011), transitional cysts have been found to be associated with a significantly higher probability of seizure in the univariate analysis. However, this association was lost after adjusting for patient age and gender as well as for number and location of the cysts. Patients with cysts in the parietal and frontal lobes were also more likely to present seizures.
5.1.2 Epileptogenesis and Neurocysticercosis
So far, the mechanism by which the calcified neurocysticercal lesions (CNL) cause seizures or epilepsy is not known (Antoniuk et al. 2001; Carpio et al. 2013; Rathore et al. 2012). This has been attributed to residual perilesional gliosis that results in chronic epileptogenic foci (Leite et al. 2000). CNL are frequently encountered in computed tomography (CT) scans of asymptomatic individuals, and studies from Latin American countries report that the majority are incidental lesions (Fleury et al. 2010; Leite et al. 2000; Kowacs et al. 2006). These observations would question the epileptogenicity of CNL.
Edema associated with CNL has also been suggested to be implicated in epileptogenicity. In fact, episodic appearance of edema surrounding the CNL after seizures has been described (Nash et al. 2008). In this study, the authors argue that episodic release of cysticercal antigens from the calcified lesions can lead to inflammation, perilesional edema, and seizures. However, it is not clear whether this edema is the cause or the consequence of seizure (Leite et al. 2000).
5.1.3 Neurocysticercosis as Etiology of Epilepsy
Although it is clear that epilepsy is the main symptom of NC, the relevance of NC as a generator of epilepsy is debated. The percentage of patients with NC in epileptic patients of endemic countries varies among studies from 14 to 54 % (Ndimubanzi et al. 2010). It is interesting, however, to note that some observations may question the causal relationship between NC and epilepsy (Sakamoto et al. 1999). Parasite location may be remote from the apparent epileptogenic region, so that there is no correlation between the NC burden of lesions and the severity of epilepsy; patients with severe refractory seizures may have only one calcified lesion, while, on the other hand, there are patients with multiple cysts or calcifications but no seizures (Carpio et al. 1998; Ferreira et al. 2002).
It is important to note that both NC and epilepsy are common diseases in most developing countries, suggesting both causal and fortuitous relationships between these pathological conditions (Sakamoto et al. 1999; Terra-Bustamante et al. 2005). In particular, in cross-sectional studies investigating the etiology of intractable epilepsy in Brazil, NC turned out to mostly represent a coexistent pathology (Pal et al. 2000; Velasco et al. 2006).
5.1.4 Risk of Seizure Recurrence in Patients with Neurocysticercosis
Some studies have reported that NC patients with acute symptomatic seizures have a good prognosis in terms of remission of seizures (Carpio and Hauser 2009; Singhi et al. 2000; Manreza 2000; Ferreira et al. 2001; Goel et al. 2010). Other studies have reported that most patients have a high risk of seizure recurrence, and suggest that prognosis improves after antihelminthic treatment (Garcia et al. 2004). Prospective cohort studies have determined a risk between 17 and 56 % of seizure recurrence after a first seizure due to NC, depending on the viability of the parasite. The risk is greater in the transitional forms and diminishes in the viable or calcified forms (Carpio and Hauser 2002; De Souza et al. 2009; Sharma et al. 2011; Thussu et al. 2008).
5.2 Extraparenchymal Neurocysticercosis (Basal Subarachnoid Space and Ventricular System)
At these locations (around 15–30 % of cases) headache and signs of elevated intracranial pressure due to the obstruction of CSF circulation are the most frequent symptoms. These occur in 88 % of the cases, in comparison with 10 % of cases with parenchymal location (Carpio et al. 1994). Inflammation of meninges can also generate cranial nerve dysfunction, chiasmatic syndrome, and cerebral infarcts secondary to vasculitis (Agapejev et al. 2007; Cárdenas et al. 2010a, b). When hydrocephalus is present (Fig. 5a), the mortality rate is high (50 %), and most patients die within 2 years after CSF shunting (Cárdenas et al. 2010a, b; Sotelo et al. 1988). This is why ventricular and basal cisternal locations are considered to be malignant forms of NC (Estañol et al. 1986).
Spinal cord cysticercosis is rare. Patients experience nonspecific clinical manifestations, such as nerve root pain or spinal cord compression syndromes, according to the level of the lesion (Alsina et al. 2002). Severe forms of NC may exceptionally occur, including cysticercotic encephalitis, and result in permanent neurological sequels, such as amaurosis.
6 Host and Parasite Factors Modulating Clinical Presentation
6.1 Age
In addition to being less common in children, NC clinical manifestations clearly vary in different age groups (Kelvin et al. 2009a, b; Rosenfeld et al. 1996). Most cases of childhood NC present mild to moderate symptomatology and single lesions (Ruiz-Garcia et al. 1997; Kelvin et al. 2011). A study specifically carried out to compare the clinical manifestations between pediatric and adult NC patients (Sáenz et al. 2006) has reported statistically significant differences: seizures were more frequent in children (80.4 % vs. 56.1 %) and intracranial hypertension and headaches were more frequent in adults (27.2 % vs. 15.2 % and 35.1 % vs. 21.7 %, respectively). Although these age-related differences seem clear, a single effect of age is difficult to demonstrate, since various confounding factors are probably involved (Kelvin et al. 2009a, b).
Most paediatric cases show a single enhancing lesion, also named solitary cysticercus granuloma (Singh et al. 2010). This lesion is a common finding in patients with newly identified seizures in developing countries. These patients have some benign and transitory clinical manifestations, predominantly partial or partial secondary generalized seizures. Single enhancing lesions tend to resolve spontaneously, without anticysticercal treatment or surgery, since the parasite is already in the degenerative phase and will eventually disappear or become calcified.
6.2 Gender
Inflammation surrounding parenchymal cysticerci is more intense in women (Kelvin et al. 2009a, b), and multiple degenerating parasites localized in the CNS parenchyma are also more frequently reported in young women. Regardless of the localization of the parasite, the inflammatory response, as expressed by CSF cellularity, is more intense in women (Fleury et al. 2010). There are significant gender and age differences in the local immune response (Kelvin et al. 2009a, b). It has been suggested that both age and gender influence the strength of the host’s immune response. The odds of having transitional cysts are higher for female patients than for males (Kelvin et al. 2011).
6.3 Genes
Clinical heterogeneity across geographical areas is well documented. Most cases from the Indian subcontinent present single degenerative lesions, whereas those from Latin America present few viable cysts (Singh et al. 2010). These differences are probably due to complex interactions between the host, parasite, and environmental factors (Singh 1997; Fleury et al. 2010). Genetic differences in T. solium cysticerci have been reported from different countries (Maravilla et al. 2008; Vega et al. 2003) and may contribute to clinical variations among countries. Genetic susceptibility to NC has been suggested on the basis of positive association of HLA-DRBII 13 with single, contrast-enhancing CT lesions (Jain et al. 1999). However, neither familial aggregation of seizures in first degree relatives of NC patients with seizures (Kelvin et al. 2009a, b) nor significant aggregation of NC cases in families have been found (Fleury et al. 2006), arguing in favor of the involvement of multiple genes.
7 Diagnosis
Diagnosis of NC cannot rely only on clinical grounds, since there are no specific clinical manifestations of NC.
7.1 Neuroimaging
Diagnosis of NC is mainly made by neuroimaging. MRI is more sensitive than CT for the diagnosis of viable and degenerating cysticerci, since it improves recognition of the perilesional edema and degenerative changes of the parasite, as well as of cysts located inside the ventricles or the subarachnoid space. However, CT is more sensitive than MRI for the detection of calcifications.
CT or MRI can identify the four developmental phases of cysticerci when located in the brain parenchyma. In the vesicular phase, the CT scan depicts circumscribed, round, hypodense areas, varying in size and number, without enhancement by contrast media (Zee et al. 2000). In the MRI, the vesicular larva appears with a CSF-like intensity signal on all sequences, with no surrounding high signal on T2-weighted images (Lucato et al. 2007; Mont’Alverne Filho et al. 2011). Both MRI and CT may show a high intensity or hyperdense, 2–3 mm mural nodule depicting the scolex, within some vesicular cysts (Fig. 4a). MRI sequences such as axial fluid attenuated inversion recovery (FLAIR) detect a significantly higher number of scolices than other sequences, which is helpful for improving the diagnosis of NC (Lucato et al. 2007). As the cyst degenerates, the contrast-enhanced CT scan shows an annular (colloidal phase) or nodular (nodular phase) enhancement surrounded by irregular perilesional edema (Fig. 4b). In this phase, the fluid content gives a slightly higher signal than CSF and is sometimes isodense with the parenchyma on MRI-T1 and/or proton density-weighted, and high signal on T2 images. The capsule shows a higher signal than the adjacent brain tissue, with thick ring enhancement on T1 images, while on T2 images there is a low ring signal surrounded by high signal lesion, due mostly to edema (Dumas et al. 1997; Zee et al. 2000). When the cyst dies it may disappear or become an inactive calcified nodule with homogeneous high density on CT or low intensity on proton-weighted MRI (Fig. 5b).
When the parasites are located in the subarachnoid space or within the ventricular system, recognition of parasites with MRI is more difficult, as parasites emit an intensity signal similar to that of the CSF, generally do not enhance after intravenous administration of contrast, and commonly lack a scolex. Thus, often only indirect signs of the presence of the parasite are available, such as the unilateral enlargement of the basal cistern (Lucato et al. 2007) (Fig. 6). Specific MRI sequences including diffusion-weighted MRI magnetization transfer ratio (MTR), 3D constructive interference in steady state (3DCISS), fast imaging employing steady state acquisition sequences (FIESTA; Fig. 6a), and FLAIR sequences have proven to be more sensitive tools to visualize the cyst wall (Braga et al. 2004; Fleury et al. 2011). In case of meningeal inflammatory process, gadolinium enhancement of MRI or contrast-enhanced CT may depict leptomeningeal thickening (Kioumehr et al. 1995) and hydrocephalus (Fig. 5a).
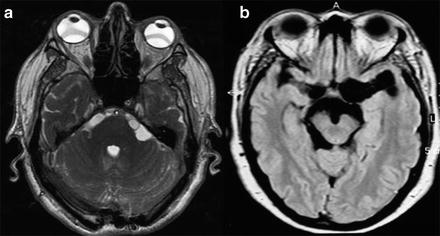

Fig. 6
MRI of extraparenchymal neurocysticercosis. (a) Cysticerci located in the cerebellopontine cistern (FIESTA sequence). (b) Cysticerci located in the basal subarachnoideal cisterns
7.2 Immunodiagnosis
No optimal immunological test for NC diagnosis is yet available. The difficulties of developing a sensitive and specific immunological test for NC diagnosis are mainly the result of the proper characteristics of the disease. An immunological test useful for medical practice must be specific in terms of CNS localization and should differentiate between viable and non-viable forms of the parasite.
Different immunological tests have been developed. The most widely used tests aim at the detection of specific antibodies. In these cases, different types of antigens have been used: crude antigens or partially purified antigenic extracts of T. solium or of the related parasites T. crassiceps or T. saginata, and recombinant or synthetic proteins. Techniques have also evolved, from complement fixation test, indirect hemagglutination, to the enzyme-linked immunosorbent assay (ELISA) and enzyme-linked immunoelectrotransfer blot (EITB) assay (Tsang et al. 1989; Brandt et al. 1992), which are the two main techniques currently in use. Also, detection of antigens by monoclonal or polyclonal antibodies using the ELISA technique has been developed (Brandt et al. 1992).
Comparisons of these tests have given divergent results, in part due to differences in methodology between studies. Despite these sources of variations, it seems that EITB in sera has higher sensibility and specificity than ELISA to detect antibodies, at least when carried out in non-endemic areas (Ramos-Kuri et al. 1992). It is also clear that sensitivity of both tests falls in cases with a single parasite, when parasites are located in the brain parenchyma or are calcified (Wilson et al. 1991; Singh et al. 1999). In CSF, performance of Ab-ELISA and EITB seems to be quite similar (Proaño-Narvaez et al. 2002; Michelet et al. 2011). Antigen detection in sera or CSF is highly specific for detecting viable extra-parenchymal parasites (Fleury et al. 2007). Another important point is the feasibility of carrying out ELISA and EITB techniques in endemic countries. In fact, while ELISA can be accomplished in 20 h and costs around $2.00 per sample, EITB assay requires almost 2 weeks, sophisticated equipment, highly skilled personnel, and its cost is up to ten times greater than ELISA (Proaño-Narvaez et al. 2002).
Despite the current immunological and imaging advances, the diagnosis of NC is still a challenge in many patients. Del Brutto et al. (2001) proposed diagnostic criteria for NC based on clinical, imaging, immunological and epidemiological features. This proposal, though not validated so far, may be useful to identify patients with parenchymal, but not extraparenchymal, forms of NC (Machado 2010). Such diagnostic criteria should be revised to incorporate current scientific knowledge, in order to achieve a new consensus on the diagnosis of NC.
8 Treatment
The treatment of NC should be individualized, based on the pathogenesis and natural history of the disease in each patient. Therapy in most cases is limited to symptomatic treatment with anti-seizure medication (ASM) for patients with seizures. Regarding duration of the ASM following an acute NC episode, some clinicians routinely continue ASM for 1 year, but shorter or longer intervals have also been recommended (Carpio et al. 2013; Takayanagui et al. 2011). It is assumed that the risk of seizures is substantial as long as there is an active ongoing process as characterized by persistence of edema around the degenerating lesion. Because of this, CT scan is a useful tool for these treatment decisions. It is appropriate to monitor cyst activity with CT scanning or MRI and to continue ASM until resolution of the acute lesion (Carpio et al. 2013). Seizures occurring in individuals after resolution of edema and reabsorption or calcification of the degenerating cyst should be considered unprovoked; in this situation, long-term ASM is warranted (Fig. 7).
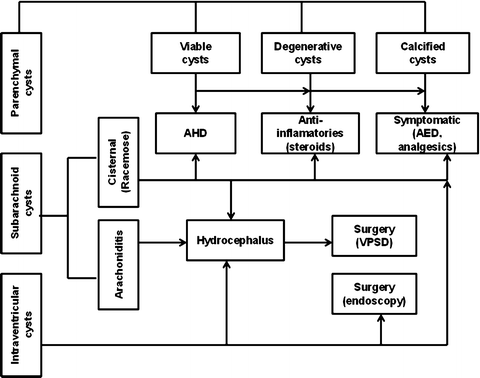

Fig. 7




Scheme for treatment of neurocysticercosis. AHD antihelminthic drugs, AED antiepileptic drugs, VPS ventriculoperitoneal shunt
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








