Neurophysiologic Basis of the Electroencephalogram
Erwin-Josef Speckmann
Christian E. Elger
Ulrich Altrup
Field potentials appear and are detectable in the space surrounding cellular elements of the nervous system. They comprise rapid waves and baseline shifts; the former correspond to the conventional electroencephalogram (EEG), and both phenomena are included in the so-called direct current (DC) potential. Field potentials are essential in the diagnosis and classification of epileptic seizures as well as in the control of antiepileptic therapy. This chapter describes the elementary mechanisms underlying the generation of field potentials and the special functional situations leading to “epileptic” field potentials.
BIOELECTRICAL ACTIVITY OF NEURONAL AND GLIAL CELLS
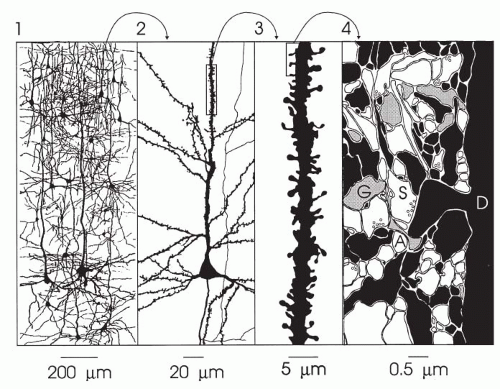
The cells of the nervous system are generally differentiated into neurons and glial cells, whose processes intermingle and form a dense, highly complex matrix (Fig. 10.1). Because the actual interactions of these cellular elements are barely recognizable in spatiotemporal dimensions, principles of their structure and function inevitably are taken into account.
Neurons
A typical neuron consists of a soma (body, perikaryon) and fibers (dendrites and axons). In functional terms, with respect to information input, the relatively short and highly arborized dendrites can be considered extensions of the soma, as reflected in their being covered by thousands of synaptic endings. Axons are relatively long and, especially in their terminal regions, branch into collaterals. These neuronal output structures carry information into the terminal regions. Information is transferred to other neurons by way of synaptic endings (1, 2, 3, 4, 5, 6, 7, 8, 9).
Neuronal function is closely correlated with bioelectrical activity, which can be studied with intracellular microelectrode recordings. When a neuron is impaled by a microelectrode, a membrane potential of approximately 70 mV with negative polarity in the intracellular space becomes apparent. This resting membrane potential, existing in the soma and all its fibers, is based mainly on a potassium-outward current through leakage channels. If the resting membrane potential is critically diminished, that is, if a threshold is surpassed, an action potential (AP) is triggered, which is based on sodium-inward and potassium-outward currents through voltage-dependent membrane channels. APs are conducted along the axons to the terminations, where they lead to a release of transmitter substances. These transmitters open another class of membrane channels in the postsynaptic neuron. Dependent on the ionic composition of the currents flowing through the transmitter (ligand)-operated channels, two types of membrane potential changes, commonly called postsynaptic potentials (PSPs), are induced in the postsynaptic neuron. When a sodium-inward current prevails, depolarization of the postsynaptic neuron occurs. This synaptic depolarization is called an excitatory postsynaptic potential (EPSP) because it increases the probability that an AP will be triggered. When a potassium-outward current or a chlorideinward current prevails, hyperpolarization of the postsynaptic neuron occurs. Because hyperpolarization increases
the distance between membrane potential and threshold, the synaptic hyperpolarization is called an inhibitory postsynaptic potential (IPSP) (10, 11, 12).
the distance between membrane potential and threshold, the synaptic hyperpolarization is called an inhibitory postsynaptic potential (IPSP) (10, 11, 12).
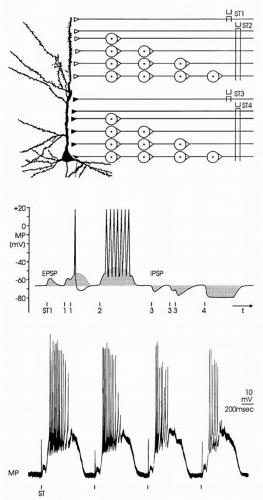
The EPSPs and IPSPs can interact with each other (Fig. 10.2). Electrical stimulation of an axon (ST1 in Fig. 10.2A) forming an excitatory synapse on a postsynaptic neuron can induce an AP at the site of stimulation. Conducted along the axon, the AP finally induces an EPSP in the postsynaptic neuron (ST1 in Fig. 10.2B). When only one synapse separates the site of stimulation from the site of EPSP generation, a monosynaptic EPSP appears. One way in which a summation of EPSP takes place is when the stimulation is repeated with an interstimulus interval shorter than the duration of the EPSP. With this temporal summation, the second EPSP can surpass the threshold and induce an AP (ST1 in Fig. 10.2B). A summation of EPSPs also can occur when monosynaptic EPSPs are evoked simultaneously at several locations on the postsynaptic neuron (spatial summation). Temporal and spatial summations are often combined with each other and are essential for information processing in the central nervous system, as when the AP reaches the target neuron by different ways. With stimulation at ST2 in Figure 10.2A, the triggered APs pass through varying numbers of relays before reaching their target. As APs are delayed with each synaptic transmission, they appear with temporal dispersion at the postsynaptic neuron and induce a long-lasting depolarization
(ST2 in Fig. 10.2B). Because many synapses are involved, such a depolarization is called a polysynaptic EPSP. When a polysynaptic network is activated repeatedly, EPSPs of considerable amplitude and duration can appear, as demonstrated by the original recording in Figure 10.2C. As with EPSPs, IPSPs can be induced both monosynaptically and polysynaptically and also are subject to temporal and spatial summation (ST3 and ST4 in Fig. 10.2A and B) (5,11,12).
(ST2 in Fig. 10.2B). Because many synapses are involved, such a depolarization is called a polysynaptic EPSP. When a polysynaptic network is activated repeatedly, EPSPs of considerable amplitude and duration can appear, as demonstrated by the original recording in Figure 10.2C. As with EPSPs, IPSPs can be induced both monosynaptically and polysynaptically and also are subject to temporal and spatial summation (ST3 and ST4 in Fig. 10.2A and B) (5,11,12).
In complex neuronal systems, EPSPs and IPSPs are often superimposed and induce long-lasting sequences of fluctuations of the membrane potential. These kinds of postsynaptic responses play a prominent role in the generation of extracellular potential fields, such as the EEG.
Glial Cells
Consisting of a soma and fibers, glial cells intermingle with the neuronal structures. Glial cell fibers are electrically coupled, building up an extended functional network (3,8,13).
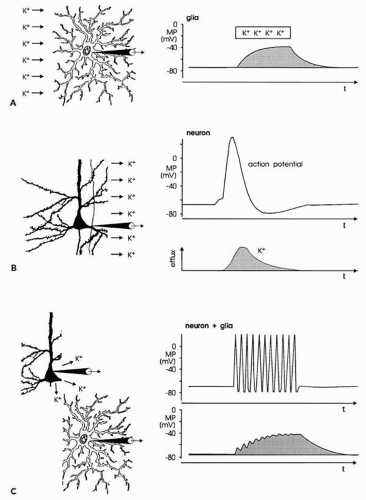
Glial cells also show a membrane potential (Fig. 10.3A). Unlike neurons, glial cells do not generate APs and PSPs.
Because their resting membrane potential is based exclusively on potassium-outward current through leakage channels, its value is close to the potassium equilibrium potential. With an increase and a subsequent decrease in extracellular potassium concentration, glial cells depolarize and repolarize, respectively (Fig. 10.3A). Changes in the extracellular concentration of other cations have only small effects on the membrane potential of glial cells (14,15).
Because their resting membrane potential is based exclusively on potassium-outward current through leakage channels, its value is close to the potassium equilibrium potential. With an increase and a subsequent decrease in extracellular potassium concentration, glial cells depolarize and repolarize, respectively (Fig. 10.3A). Changes in the extracellular concentration of other cations have only small effects on the membrane potential of glial cells (14,15).
Glial cells and neurons are functionally linked by way of the extracellular potassium concentration (Fig. 10.3B and C). As mentioned, neuronal APs are associated with an outflow of potassium ions (Fig. 10.3B). Thus, with an increase in the repetition rate of neuronal APs, the extracellular potassium concentration increases, resulting in depolarization of glial cells adjacent to the active neurons (Fig. 10.3C) (11,14, 15, 16).
PRINCIPLES OF FIELD POTENTIAL GENERATION
Changes in membrane potential of neurons and glial cells are the basis of changes in extracellular field potential. The mechanisms involved can be described as follows: (a) primary transmembranous ion fluxes at a restricted membrane area of cells and consequent localized membrane potential changes; (b) development of potential gradients between sites of primary events and the remaining areas of the membrane; and (c) secondary ion currents because of the potential gradient along the cell membrane in the intracellular and extracellular spaces. The secondary current flowing through the extracellular space is directly responsible for the generation of field potentials (9,17). Because EPSPs and IPSPs are important in the generation of the EEG findings, the processes are explained in greater detail using the examples of an excitatory synaptic input (2,12,18,19).
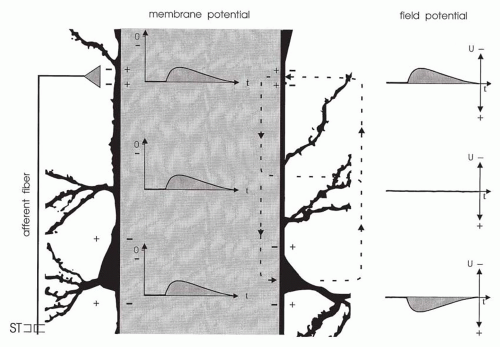
A vertically oriented neuronal element, shown schematically in Figure 10.4, is impinged on by a single excitatory synapse whose afferent fiber can be stimulated. The resulting net influx of cations leads to depolarization of the membrane, that is, to an EPSP. Consequently, a potential gradient exists along the neuronal membrane and evokes an intracellular and extracellular current flow. As a result of the intracellular current, the EPSP spreads electrotonically; the extracellular current induces field potentials. The polarity depends on the site of recording. The electrode near the synapse “sees” the inflow of cations (a negativity), whereas the electrode distant from the synapse “sees” the outflow of cations (a positivity). Between the two electrodes is the reversal point of the field potentials (12,20).
Corresponding effects occur with the generation of IPSPs. Activation of an inhibitory synapse induces an outflow of cations or an inflow of anions at the synaptic site. In this way, the membrane potential is increased at the synaptic site, and a potential gradient develops along the cell membrane, similar to that described for EPSPs. The potential gradient evokes a current flow from the synaptic site to the surrounding regions of the membrane. Compared with EPSPs, the extracellular current flow is inverted, as is the polarity of field potentials. Thus, the electrode near the synapse “sees” a positivity and the electrode distant from the synapse a negativity.
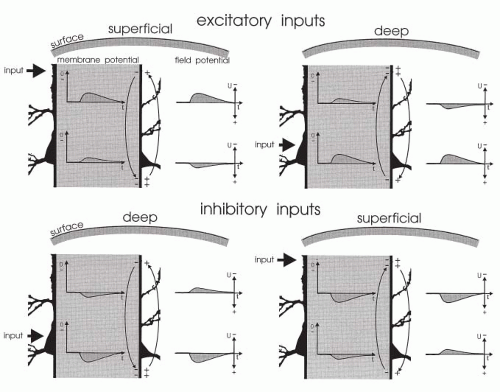 Figure 10.5 Generation of field potential in the neocortex by excitatory and inhibitory synaptic inputs reaching the superficial and deep parts of perpendicular pyramidal neurons. The intracellular space is extended (hatched areas). Changes in membrane potential and in the corresponding field potential are given in the intracellular and extracellular spaces, respectively. Locations of active inputs are indicated (heavy arrows). EPSP and IPSP, excitatory and inhibitory postsynaptic potentials, respectively. Excitatory inputs: With superficial excitation, an inward current generated an EPSP in upper and lower regions. Because of the direction of the extracellular current flow (light arrows), the field potential had negative polarity at the surface and positive polarity in the deep recording (cf. Fig. 10.4). With deep excitation, the current flow—and the field potentials—had inverse direction to that elicited by superficial excitation. Inhibitory inputs: With deep inhibition, an outward current generated an IPSP in lower and upper regions. Because of the direction of the extracellular current flow (arrows), the field potential had positive polarity in the deep recording and negative polarity at the surface. With superficial inhibition, the direction of current flow was inverse to that seen with deep inhibition; the field potentials were inverted as well. Differences in the shape of the various potentials were caused by the electrical properties of the tissue. |
Field potentials are generated by extracellular currents, and their polarity depends on the direction of the current as well as on the positions of the extracellular electrodes. Figure 10.5 illustrates the generation and polarity of field potentials, as elicited by excitatory and inhibitory inputs to superficial and deep regions of vertical neuronal elements. Negative field potentials at the cortical surface may be based on superficial EPSPs as well as on deep IPSPs, and positive field potentials at the surface may be based on superficial IPSPs as well as on deep EPSPs (Fig. 10.6) (12,19,20).
POTENTIAL FIELDS IN NEURONAL NETWORKS
Many neuronal elements contribute to the extracellular currents that generate field potentials recorded at the surface of central nervous system structures. The spatial arrangement of the neuronal elements and the positions of the recording electrodes play an essential role in establishing and detecting extracellular potential fields (2,12,21).
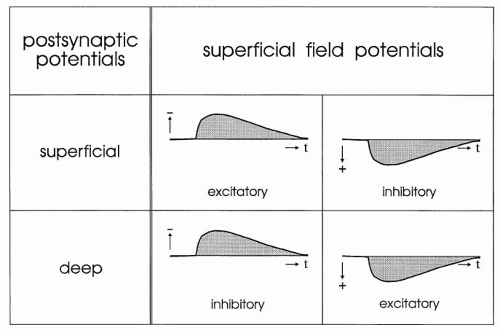 Figure 10.6 Synopsis of the synaptic processes underlying the generation of superficial field potentials in the cerebral cortex. Different mechanisms may lead to uniform superficial field potentials. |
Two principal types of neuronal arrangements can be identified (Fig. 10.7). In the parallel type, the somata are in one layer and the dendrites are in opposite layers (Fig. 10.7A). In the other type, the somata are in the center of a pool and the dendrites extend to its periphery (Fig. 10.7B). The first arrangement is realized in the cortex and the second in brainstem nuclei.
The two neuronal arrangements build up the so-called open and closed fields. In open fields, one electrode (E2 in Fig. 10.7A) largely integrates the potentials of the population (i.e., it is near the zero potential line), and the other electrode (E1 in Fig. 10.7A) sees only the positive or negative field, permitting the recording of a field potential. In closed fields, external electrodes do not see significant potential differences because the current flows within the pool compensate for each other (Fig. 10.7B) (2,21).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree