INTRODUCTION
NEUROPSYCHIATRIC SYMPTOMS IN PARKINSON’S DISEASE
A variety of neuropsychiatric symptoms (NPS), including depression, anxiety, psychotic symptoms, apathy, and impulse-control disorders (ICDs), occur in up to 60% of patients with Parkinson’s disease (PD) (1). NPS have a significant impact on patients and their caregivers, as well as on the health-care system (2). However, they are underrecognized and undertreated (3), and this is often due to patient-related factors (e.g., incomplete understanding of mental health problems), access-to-care issues, as well as a lack of time and knowledge among many clinicians (3,4).
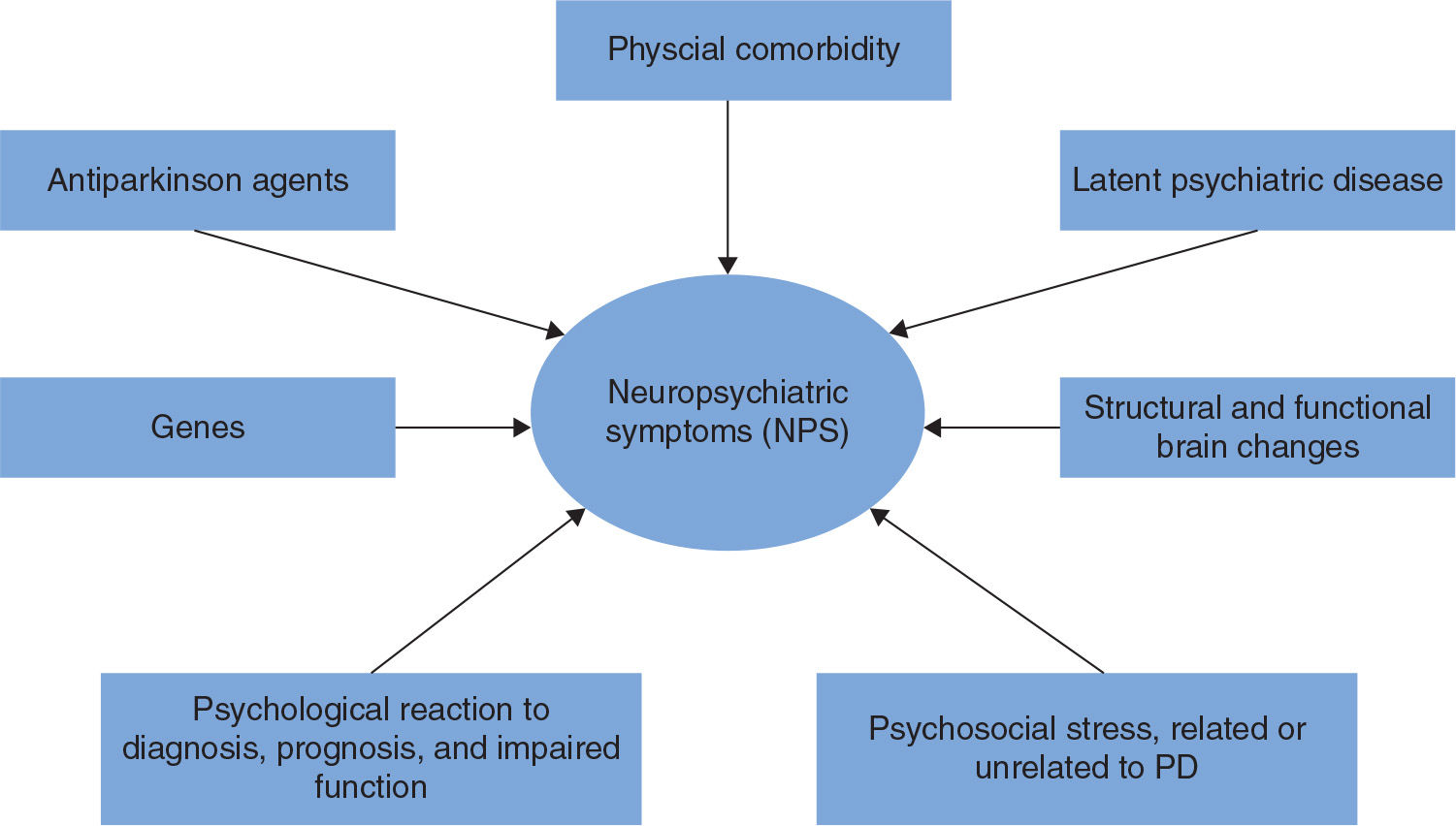
Although little is known regarding causal factors, phenotypic variation suggests that a variety of factors contribute to NPS in PD. Some NPS, such as depression and anxiety, are common in the elderly in general as well as in other brain diseases, whereas other symptoms are more characteristic for PD and related disorders, such as visual hallucinations, misidentification syndrome, REM sleep behavior disorder (RBD), and ICDs. RBD will be discussed in Chapter 42 (Movement Disorders in Systemic Disease). The neuropsychiatric profile of PD thus differs from that of patients with Alzheimer’s disease (AD) (5) and even from other diseases of the basal ganglia (6). Therefore, PD-specific factors (e.g., involvement of specific brain regions and the role of dopamine replacement therapy [DRT]), nonspecific psychological and social reactions, and genetic factors (7,8) all seem to play a role for the development of NPS in PD (Fig. 13.1).
There is evidence that the profile, frequency, and severity of NPS tend to increase with cognitive impairment and dementia in PD (PDD). In one study, 89% of PDD patients had at least one NPS, and 60% had NPS of clinical significance (9). Hallucinations and apathy are symptoms which typically increase in frequency and severity when cognition declines, whereas a syndrome with exclusively sleep or mood symptoms seems to be more common in PD without dementia (10). Fig. 13.2 demonstrates three studies showing the percentage of patients with positive Neuropsychiatric Inventory (NPI) item scores across the spectrum of cognitive impairment in PD. Although direct comparison across different cohorts is subject to selection bias, the tendency toward more frequent NPS with worsening cognition is evident. The co-occurrence of cognitive impairment and NPS is likely due to common pathologies such as neocortical morphologic changes (e.g., synuclein, tau, and amyloid pathology) and neurotransmitter changes (cholinergic and monoaminergic changes in addition to dopaminergic deficits). In addition, subjective cognitive decline may lead to anxiety and depression, and cognitive decline may influence the interpretation of various sensory stimuli, (e.g., visual disturbances and illusions may more easily be interpreted in a psychotic context in someone with dementia). In this chapter, we will discuss the epidemiology, etiology, clinical features, diagnostic procedures, and management of some of the most important NPS in PD, including depression, anxiety, psychotic symptoms, apathy, and ICDs.
NONMOTOR FLUCTUATIONS
Before discussing the different NPS, we want to stress that although NPS are usually fairly consistent in their presence over a period of time, this is not always the case. Many PD patients on chronic levodopa treatment may experience motor fluctuations (MFs), which involve “off” periods characterized by worsening parkinsonism. Recent research has shown that the majority of patients with MFs also experience nonmotor fluctuations (NMFs), which are typically a mix of psychiatric (e.g., anxiety, fatigue, and dysphoria) and cognitive (e.g., slowness of thinking, confusion, poor concentration, word finding problems, impaired memory) symptoms, as well as autonomic symptoms (e.g., urinary incontinence and profuse sweats) (11,12). In one of the most detailed studies of NMFs, 100% of the patients with MFs also had at least one type of NMF, symptoms such as anxiety and fatigue were among the most common NMF and occur in “off” periods. Of note, in 28% of the patients, the NMFs were deemed to be more disabling than MFs (11). However, there is not always a correlation between motor and nonmotor symptoms (13,14). Clinical experience suggests that DRT may improve NMFs, but it remains to be seen if treatments shown to reduce severity or time of MFs also lead to improvements in severity or duration of NMFs, although psychiatric NMFs seem to respond less well than cognitive and autonomous NMFs (15).
DEPRESSION
Depressive symptoms are among the most common NPS in PD (3,16) and are associated with reduced motor function, impaired cognitive functioning, lower health-related quality of life and higher caregiver distress (17,18). Depression is also a common symptom of other diseases and in elderly people in general; however, studies of depression in different populations suggest that this mood disorder is relatively more common in patients with PD than in healthy elderly and in patients with other chronic and disabling diseases (19). The key characteristics of depression in PD are similar to those observed in major depressive disorder: low mood and lack of interest or pleasure (anhedonia), one of which is required for a diagnosis of depression in most classifications (20). Other features of depression can be present in varying combinations, but many of these, such as altered appetite or sleep, weight change, loss of libido, psychomotor retardation, reduced memory, apathy, and loss of energy, can overlap with the motor and autonomic symptoms of PD, making diagnosis of depression in PD complicated (21). Other features of depression include feelings of guilt or worthlessness and suicidal ideation, but these symptoms may be less common in patients with PD compared to depressed patients without PD, whereas symptoms such as concentration and sleep disturbance may be more common in depressed PD patients (22,23).
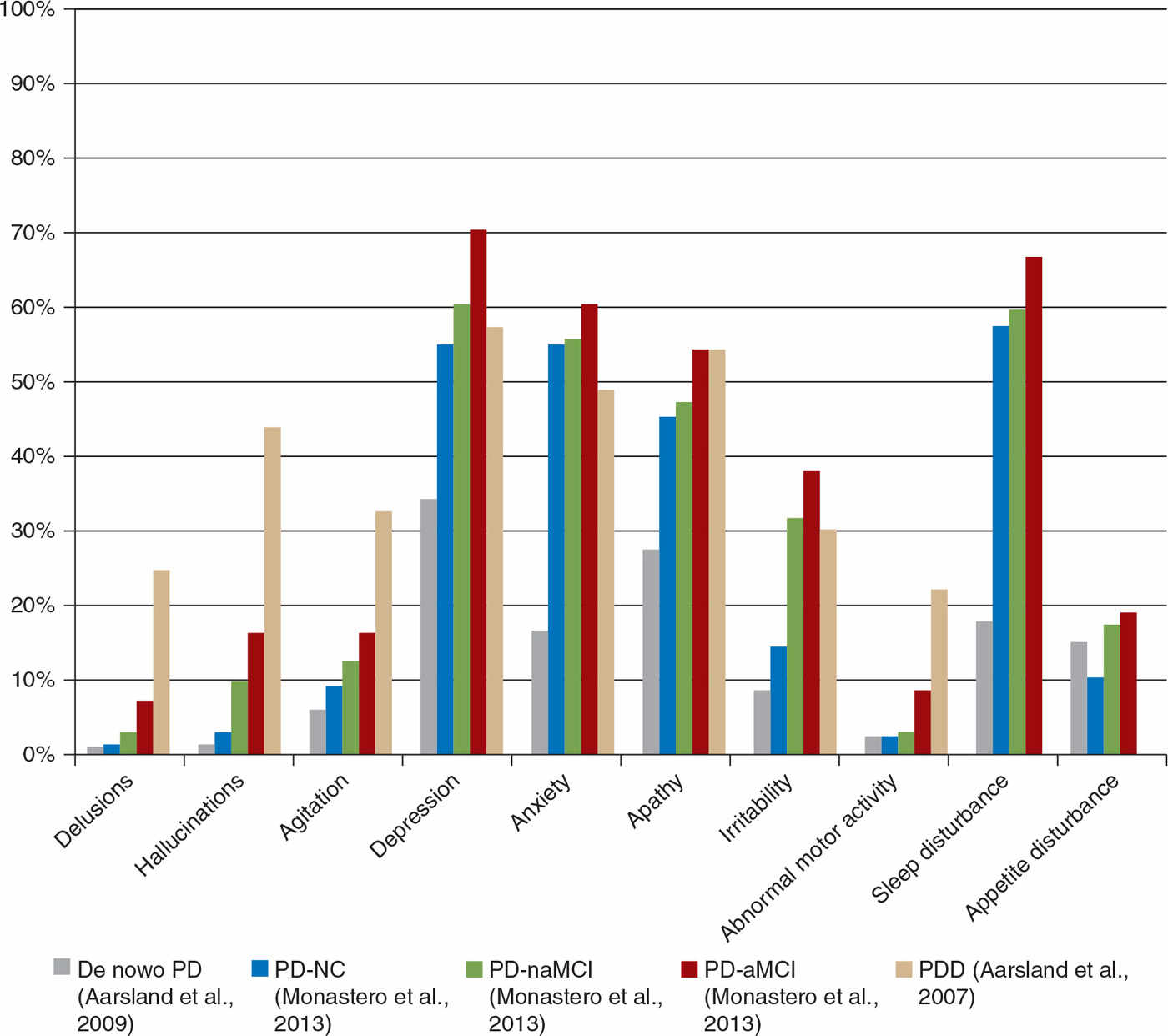
Figure 13.2. Proportion (%) of PD patients with a nonzero score on neuropsychiatric inventory (NPI) items by cognitive status. Data is lacking on the percentage of PDD patients suffering from sleep disturbance or appetite disturbance. PD-NC, PD not cognitively impaired; PD-naMCI, PD with nonamnestic mild cognitive impairment; PD-aMCI, PD with amnestic mild cognitive impairment; PDD, PD with dementia.
EPIDEMIOLOGY OF DEPRESSION IN PD
Approximately 35% of PD patients suffer from clinically relevant depressive symptoms (3,16). However, there are wide variations reported in the frequency of depression in PD, ranging from 2.7% to more than 90%, depending on criteria and assessment of depression in addition to sample selection. A systematic review reported an average prevalence of major depressive disorder of 17%, minor depression was present in 22% of the PD patients, and dysthymia in 13% (16). These findings are consistent with the large GEPAD study reporting depression in 24% (24). Depressive symptoms may occur prediagnosis, and in a minority of patients already at the time of diagnosis, although the frequency tends to be higher in more advanced PD. Depression seems to increase in parallel with cognitive decline. For example, in one study, rates of depression (based on a nonzero score on the NPI dysphoria item) (25) were 70% in amnestic mild cognitive impairment (MCI), 60% in nonamnestic MCI, and 55% in normal cognition (26) (see Fig. 13.2). Similarly, in the GEPAD study, the frequency of depression (defined as a score ≥14 on the Montgomery and Åsberg Depression Rating Scale) increased with disease stage, and was higher in PDD (44%) than in nondemented (18%) patients (27).
DIAGNOSIS OF DEPRESSION IN PD
Symptoms of motor parkinsonism, cognitive impairment, and apathy may overlap with symptoms of depression. In addition, cognitive impairment and apathy can reduce awareness and influence the capacity to communicate the symptoms of depression. Thus, there is a risk both for under- and overdiagnosis of depression in PD. Commonly used diagnostic criteria for depression such as the Diagnostic and Statistical Manual of Mental Disorders (28) can be difficult to apply to PD patients, since they point that only symptoms that are not caused by a medical condition or a direct effect of a substance can be considered as a symptom for depression. In PD, it can be a challenge if somatic symptoms, including altered sleep or appetite, weight loss, energy disturbance, psychomotor retardation, and reduced memory, are a result of PD or should be counted as a depressive symptom. Accordingly, since application of these diagnostic criteria may result in underdiagnosis of depression in PD, a NINDS/NIMH working group have made some recommendations to establish diagnostic criteria for depression in PD, underlining that such symptoms should be counted as representing depression regardless of their overlap with PD (Table 13.1) (24). The use of standardized depression scales is recommended, both questionnaires as well as clinical interviews. Commonly used depression scales include Geriatric Depression Scale, Hamilton Depression Scale; Montgomery–Åsberg Depression Rating Scale, and Hospital Anxiety and Depression Scale have been shown to have good psychometric properties also in PD and can facilitate the diagnosis of depression in a patient with PD (8).
ETIOLOGY OF DEPRESSION IN PD
The underlying mechanisms of depression in PD are complex and are still not fully understood, and several theories have been proposed. Psychological theories argue that depression is secondary to the stress and coping of a chronic disabling disease. However, the high prevalence of depression in PD cannot completely be explained by psychological theories. PD patients have more depressive symptoms than patients with other chronic disabling diseases (29). Neurobiologic theories suggest that depression is the result from the neurodegenerative processes that manifest in PD. Evidence from a variety of sources, including neuroimaging studies, cerebrospinal fluid (CSF) analysis, and studies of brain tissue, suggests that primary degeneration of dopaminergic mesocortical and mesolimbic neurons that project into the dopaminergic frontal lobe, as well as of noradrenergic limbic and brainstem structures, is associated with depression in patients with PD (21,30). The neurotransmitters dopamine, noradrenalin, and serotonin are known to be involved in the regulation of mood and in depression in the general population (21). In addition to the stress of a chronic disabling disease and the neurodegenerative processes that manifest in PD, environmental factors, as is the case in the general population, may contribute to the development of depression (31).
MANAGEMENT OF DEPRESSION IN PD
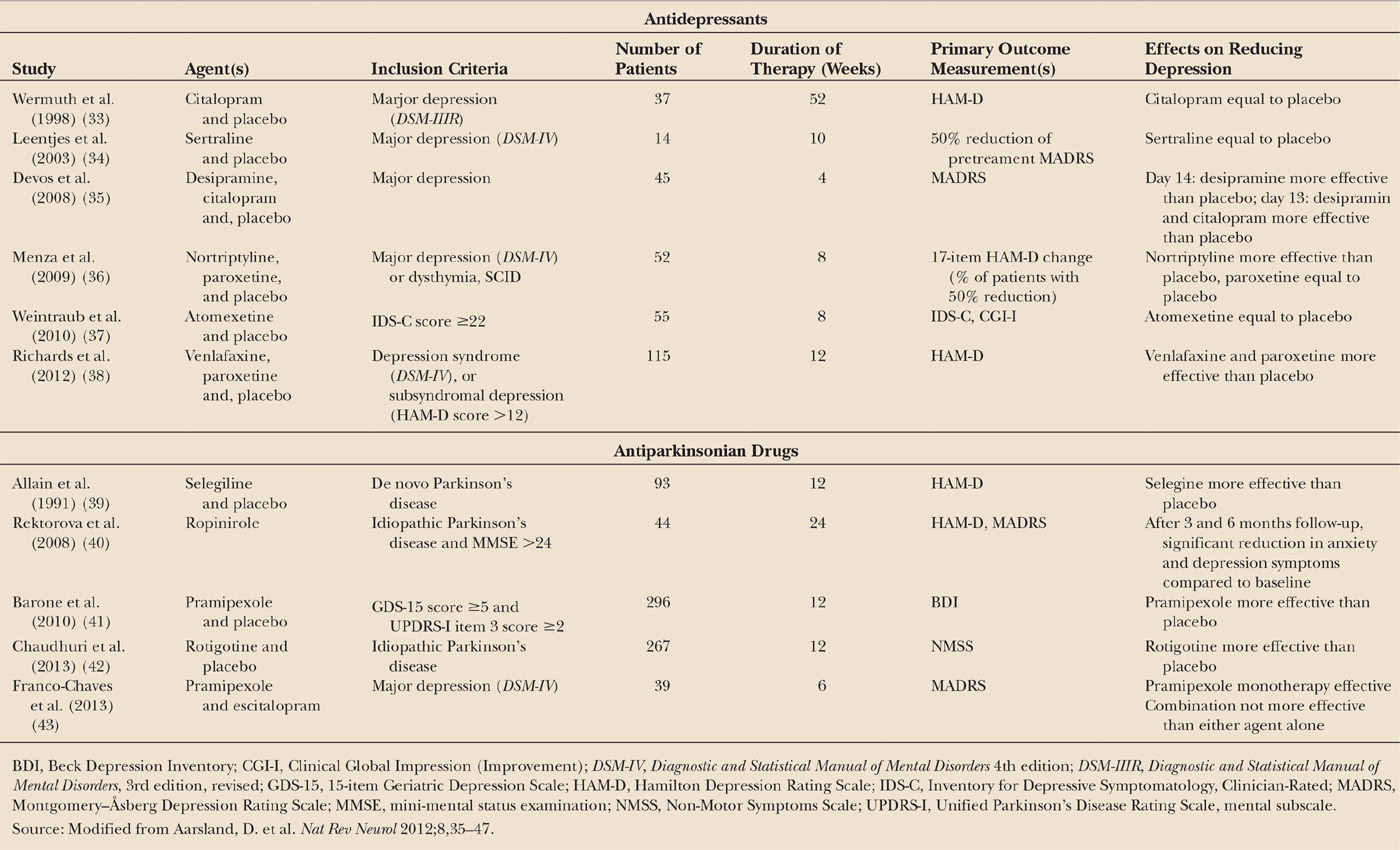
Despite the high prevalence and the impact of depression in PD, there is evidence that depression is undertreated, with one study suggesting that only 20% of the patients received any form of treatment (32). The management of depression in PD is complicated due to the challenge of diagnosing depression, the higher sensitivity to side effects of the pharmacologic therapies, and the reluctance to accept the diagnosis of depression. Potential treatment options are pharmacologic therapies (Table 13.2), including antidepressants therapies and antiparkinson drugs, and nonpharmacologic therapies (Table 13.3), including cognitive behavioral therapy (CBT), electroconvulsive treatment (ECT), and repetitive transcranial magnetic stimulation (rTMS).
| NINDS/NIMH Working Group Recommendations for Diagnosing Depression in Parkinson’s Disease |
Recommendations
1. Diagnosis of depression in PD should be based on the DSM diagnostic criteria by using an inclusive approach (i.e., symptoms should be counted as representing depression regardless of their overlap with PD or other medical conditions).
2. Decreased interest should be eliminated as a core symptom of depression.
3. The DSM exclusion criteria “due to the effects of a general medical condition” should be omitted.
4. The timing of the diagnostic assessment should be defined, during “off” periods or during “on” periods.
5. Subsyndromal depression should be considered in research studies as a distinct diagnostic category.
6. For cognitive impaired patients, informants closely related to the patient are necessary for the symptoms ratings.
Source: Modified from Marsh L, et al. Mov Disord 2007;21:148–158.
| Nonpharmacologic Treatment Modalities for Depression in Parkinson’s Disease (Randomized Trials) |
Antidepressant Therapies
Selective serotonin reuptake inhibitors (SSRIs) are the most common antidepressant class to be used in PD. However, until recently there has been little robust evidence supporting the use of these drugs. Several early clinical trials did not find a significant difference between antidepressants and placebo (see Table 13.2), and one meta-analysis found no significant effects of antidepressant therapies for depression in PD (44). In a more recent meta-analysis, which included the two recent positive trials (see below), the authors reported a moderately sized, but nonsignificant, pooled effect size of 0.71. They concluded that the lack of a statistically significant difference was likely due to the low number of available placebo-controlled studies (45). In another recent review, based on five placebo-controlled studies (33–36,38), the overall risk ratio (RR) for response was not statistically significant, 1.36 (95% CI 0.98–1.87). However, in two sensitivity analyses, including only the studies with the best design, there was a significant benefit of antidepressant therapies (46). The three most recent and methodologically robust trials are discussed below.
The first level-1 evidence of an antidepressant in PD included 55 patients with a depressive disorder, as determined by the DSM-IV criteria. Patients treated with nortriptyline, a tricyclic antidepressant with predominantly norepinephrine uptake inhibition, had a statistically significant reduction in depression (effect size of 1.20) compared with patients who received placebo, whereas no effect was found in the group receiving paroxetine, an SSRI. Treatment with nortriptyline is frequently associated with adverse effects in patients, such as sedation, hypotension, and even worsening of cognition, but the patients in this study who received nortriptyline did not report more of these adverse events compared with placebo group (36). Of note, patients who showed an improvement in depression scores also had additional benefit in terms of improved quality of life and social functioning. Improvement in depression was maintained in patients who were treated with nortriptyline over a 16-week extension to this study (47); patients who received the active drug had a lower risk of relapse during the extension phase than those on placebo. Since depression can induce cognitive impairment, one may hypothesize that improvement of depressive symptoms may be accompanied by cognitive improvement. However, in this study, cognition did not improve in the patients treated with nortriptyline.
In another important study, the antidepressant effects of venlafaxine, a serotonin and norepinephrine reuptake inhibitor (maximum daily dosage 225 mg) and paroxetine (maximum daily dosage 40 mg) were compared to that of placebo over the course of 12 weeks (38). Significant effects compared to placebo were found for both paroxetine and venlafaxine on the depression rating scale score, but not when the scale was dichotomized to assess response versus no response (response is defined as a ≥50% reduction in depression rating scale score). There were positive effects on sleep in the venlafaxine group, but no effects on cognition or motor symptoms in either groups.
A small study that investigated the effect of atomoxetine, a selective norepinephrine reuptake inhibitor, in patients with PD showed no significant antidepressant efficacy versus placebo (37). However, when the definition of efficacy was more liberally applied (a >40% decrease in depression score instead of a >50% decrease), a significantly higher proportion of patients had improved depression when treated with atomoxetine (31.8%) compared to patients who received placebo (9.5%). Of interest, global cognition and daytime sleepiness improved after treatment with atomoxetine, and the drug was well tolerated.
In conclusion, the emerging evidence suggests that dual-acting antidepressant drugs (those that act on both serotonergic and noradrenergic systems) such as nortriptyline and venlafaxine, might be useful in the treatment of depression in PD, but supporting evidence from meta-analyses is lacking. The evidence for SSRIs, the most commonly used drug class for depression in PD, is inconsistent, although positive effect of paroxetine was found in one study (40). Further research into the effect of antidepressant agents on depression in patients with PD is warranted.
Dopaminergic Agents
The potential involvement of dopamine in the pathogenesis of depression in patients with PD suggests that dopaminergic treatment might improve depression in these individuals, and several trials of the effects of dopamine agonists on mood have been reported (see Table 13.2). In a large, placebo-controlled, clinical trial treatment of patients with the dopamine agonist, pramipexole (given at a mean dose of 2.18 mg per day) led to a significant reduction in depression scores compared with scores of patients who were treated with placebo. The actual improvement in depression was quite small (possibly related to the generally mild depression severity of patients included in the trial), but seemed to be independent of motor improvement (41). Pramipexole was well tolerated, and the added benefit of this drug in terms of improving motor function makes it a tempting choice as a first-line antidepressant in patients with PD and depression. In a post hoc analysis of a placebo-controlled trial of rotigotine with 267 patients, several domains of the Non-Motor Symptom Scale (NMSS) improved significantly in the rotigotine group, including four of seven items in the Mood/Apathy domain (42). Uncontrolled supportive evidence also exists for ropinirole (40). Bupropion is a dual-acting inhibitor of dopamine and noradrenaline reuptake, which thus might be particularly relevant for the treatment of depression in patients with PD (49), but systematic evidence is lacking. Finally, in a randomized, double-blind, placebo-controlled study, the monoamine oxidase B inhibitor, selegiline, exerted a delayed mood-enhancing effect in patients with PD who were not clinically depressed (39).
Nonpharmacologic Treatment Modalities (Table 13.3)
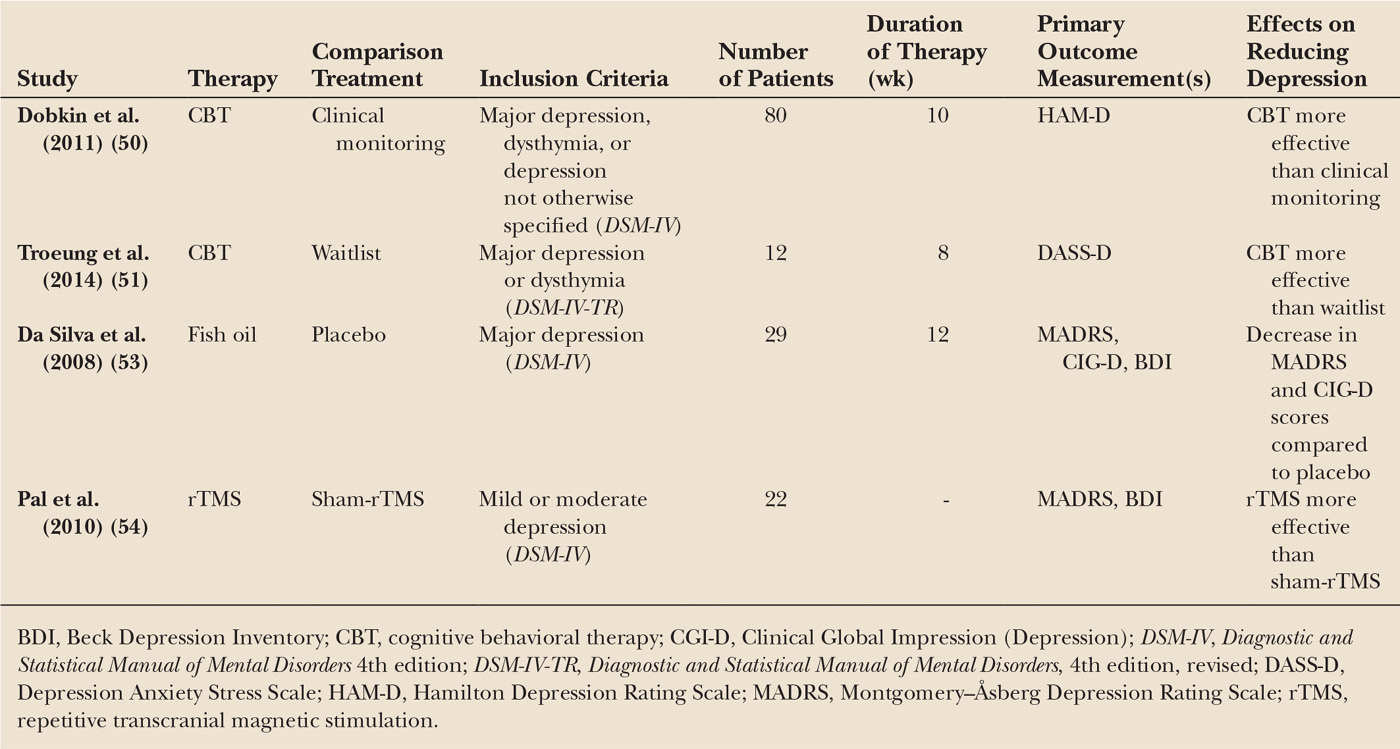
CBT is a frequently used technique to treat anxiety and depression in the general population. Maladaptive behavioral patterns are addressed, with the underlying premise that it is the meaning ascribed to the social situations which is the key to patient’s suffering. Recent evidence from small randomized trials suggests that it is also effective for depression in PD (50,51), with better executive functioning being a significant and independent predictor of response to treatment effect (52).
Other small trials showed promising effects on depression and anxiety of fish oil (containing omega-3 fatty acids) (53) and left prefrontal rTMS (54).
Some reports suggest that ECT has antidepressant effects in PD (21,55), but there is a risk for adverse cognitive effects after ECT, which is likely higher in PD patients than in patients without brain diseases. Of note, due to the dopamine-enhancing effect, ECT may also improve motor symptoms of PD. Deep brain stimulation (DBS) is also explored as a treatment option for treatment-resistance depression (56). DBS of the subthalamic nucleus (STN) has already proven to reduce motor symptoms in patients with severe, refractory PD, essential tremor, and dystonia. However, its effect on psychiatric functions is still controversial. Some studies found improvements in depressive symptoms after bilateral DBS of the STN compared with the preoperative condition, other studies found no improvement or even worsening of the depressive symptoms (57).
ANXIETY
Compared to depression, much less is known about anxiety in PD. In one large cohort, 34% of the PD patients met a diagnosis of one anxiety disorder, general anxiety disorder (GAD) being the most common diagnosed condition, followed by panic attacks and phobias (58). GAD is characterized by excessive and uncontrollable worry accompanied by physiologic arousal, for example, muscle tension. Typically, PD patients report worry about disease progression and everyday worries such as finances (58). Panic attacks are also common, such as increased heart rate, chest pain, dizziness, difficulty breathing, sweating, and concern over “losing control” or “going mad,” and may lead to fear over their consequences and avoidance behavior. Patients with social phobia fear the perceived social consequences resulting from PD symptoms manifesting themselves in public (58).
However, similar to depression, there is evidence that the DSM system does not adequately capture the anxiety morbidity in PD, since many patients with significant anxiety symptoms on rating scales do not fit in any of the DSM anxiety categories (28). In addition, as is the case in the non-PD elderly population, anxiety is strongly linked with symptoms of depression, which has been demonstrated in two large series (59,60). Interestingly, persistent anxiety was strongly associated with depression, suggesting a distinct affective syndrome in PD, whereas episodic anxiety was less associated with depression but rather with symptoms of phobia (60), possibly related to PD patients awareness of having PD in social contexts. This distinction in persistence and episodic anxiety suggests that a structured psychiatric interview should be administered to those with high scores on affective symptom rating scales. Of note, anxiety symptoms are also related to motor symptoms, are more common in patients with MFs, and seem to occur more often in the off condition (please see section on NMFs), although in the majority of cases anxiety symptoms seem to occur without any temporal relationship with specific motor states (61).
ETIOLOGY OF ANXIETY IN PD
The degeneration of the locus coeruleus occurring early in the course of PD (62) might play a role in the development of anxiety in these patients (63), and this hypothesis received some support from findings in a PET study (64): lower binding of [11C]RTI-32, with affinity to both dopamine and noradrenaline, was found in the locus coeruleus of depressed compared with nondepressed patients. In addition, the negative correlation found between locus coeruleus [11C]RTI-32 binding and severity of anxiety in PD supports a direct role for norepinephrine in the pathophysiology of anxiety in PD.
MANAGEMENT OF ANXIETY IN PD
There have been no controlled anxiety treatment studies in PD, but some of the studies of depression have reported a secondary benefit for anxiety symptoms during antidepressant treatment. There was a significant benefit of nortriptyline over placebo on an anxiety scale, and a trend toward significant benefit of paroxetine (36).
For patients who experience anxiety as part of an “off“ state, PD medication adjustments can be made in an attempt to decrease the duration and severity of these episodes (please see section on NMFs). However, many patients require treatment with benzodiazepines, although this medication class must be used cautiously in PD patients because of their propensity to increase sedation, gait imbalance, and cognitive impairment (65). Several preliminary and some controlled studies suggest that CBT are useful in PD; and recently recommendations for adapting CBT for treating symptoms of anxiety and depression in PD were provided (66). This was supported in a recent randomized waitlist-controlled study of PD patients with anxiety and/or depression, which showed a large and statistically significant effect size of CBT (d = 1.12) (51). Potential barriers to CBT in the PD population are the presence of cognitive impairment and difficulty attending regular in-person visits. Therefore, telephone- and Internet-based CBT are gaining in popularity.
PSYCHOSIS
Psychosis symptoms in PD include hallucinations and delusions, and the occurrences of these symptoms in PD patients are predictors of nursing home placement (67) and mortality (68). Visual hallucinations are the most typical manifestation. The patient often sees people, animals, or static objects. Visual hallucinations tend to occur intermittently either at the end of the day or in dim lighting conditions and can last from seconds to several minutes. Frequency in occurrence may vary from rare or a few times in a week, but in more advanced stages of the disease, it can occur many times in a day or even be persistent. Insight can be lost as the disease worsens or when the cognitive impairment ensues. Occasionally, the hallucinations can be of a malignant theme. In addition, other visuoperceptual disturbances can occur, including illusionary experiences, sensations of movement in the periphery (passage hallucination), and sensations of presence (extracampine or “social” (69) hallucination). Hallucinations in other modalities (auditory, tactile, and olfactory) can also occur but are less common (67,70).
Delusions manifest when insight is compromised, so are often associated with degree of cognitive impairment. Delusions in PD tend to be paranoid (i.e., believing that someone is out to harm you) in nature. Other phenomena, including delusional misidentification (e.g., Capgras and Fregoli syndromes) (71,72), are discussed below.
EPIDEMIOLOGY OF PSYCHOSIS IN PD
Psychotic symptoms affect up to 60% of PD patients long term (68,70,73), although the precise point prevalence is not clear. Recent development of rigorous criteria will prove helpful in defining and investigating the epidemiology and etiology of these symptoms (Table 13.4) (74).
Complex visual hallucinations are present in 7% to 25% of PD patients, but rise to 41% to 87% in PDD patients (67,75). Regarding delusions, determination of their true prevalence in PD is difficult as their occurrence is often conflated with visual hallucinations. However, it appears that isolated delusions without hallucinations (prevalence of 4%–5%) probably occur less commonly than hallucinations (68,70,76).
RISK FACTORS OF VISUAL HALLUCINATIONS
Psychosis can manifest across the disease spectrum in PD, even in the earliest stages of the disease (77), although typically it occurs later in the disease course (68). Risk factors for visual hallucinations include disease duration (78,79), severity of the disease (71), ocular disorders (80), higher age of PD onset (68), depression (81), and RBD (82). Another risk factor for visual hallucinations is a reduction in cognitive performance (77). The role of cognitive impairment, however, is complex, and on the other hand, the occurrence of visual hallucinations in PD may be a harbinger of subsequent cognitive decline, or in the presence of preexisting dementia can lead to more rapid decline (83,84). PD hallucinators tend to have more executive dysfunction, poorer sustained attention, and worse visuoperceptual function compared with nonhallucinators (81,85), and impairments in attention (85,86) and visuoperceptual function (87) are central to a number of etiologic models of visual hallucinations (88,89), so it may be that there are common neuropathologic processes leading to the occurrence of both hallucinations and cognitive decline.
| NINDS–NIMH Diagnostic Criteria for Psychosis in PD |
Diagnostic Criteria | ||
Criterion A | Characteristic symptoms | Presence of at least one of the following symptoms: • Illusions • False sense of presence • Hallucinations • Delusions |
Criterion B | Primary diagnosis | UK Brain Bank criteria for PD |
Criterion C | Chronology of the onset of symptoms of psychosis | The symptoms in criterion A occur after the onset of PD |
Criterion D | Duration | The symptoms in criterion A are recurrent or continuous for 1 month |
Criterion E | Exclusion of other causes | The symptoms in criterion A are not better accounted for by another cause of parkinsonism such as dementia with Lewy bodies, psychiatric disorders, or a general medical condition, including delirium. |
Source: Modified from Ravina et al. (2007). Diagnostic criteria for psychosis in Parkinson’s disease: Report of an NINDS, NIMH work group. Movement Disorders, 22 (8), 1061–1068. | ||
ETIOLOGY OF PSYCHOSIS IN PD
The role of dopamine and DRT in the etiology of psychosis in PD remains controversial. While dopamine agonists seem to be associated with an increased risk of visual hallucinations (79), it is notable that dementia with Lewy body (DLB) patients can experience similar visual hallucinations without exposure to DRT, and there are historical reports of late-stage PD patients in the pre-levodopa era experiencing hallucinations. Similarly, intravenous levodopa in hallucinating PD patients does not precipitate hallucinations (90). This, along with the observations that anticholinergics can exacerbate visual hallucinations (91) and cholinesterase inhibitors (ChEIs) may improve them, suggests that a pure dopamine-based explanation for PD psychosis is unlikely, and that dysregulation in other neurotransmitter systems (e.g., cholinergic and serotonergic systems) also play a role (92).
MANAGEMENT OF PSYCHOSIS IN PD
PD psychosis can be difficult to treat, and there are no systematic studies evaluating nonpharmacologic interventions, although a small study has suggested benefit for ECT in patients refractory to antipsychotics (93). Practically, removal of anticholinergics, discontinuation of dopamine agonists, and reductions in levodopa load may help ameliorate psychotic symptoms, particularly in the earlier stages of the disease, and a specific order of withdrawal has been suggested beginning with anticholinergic agents and amantadine, through to dopamine agonists/COMT inhibitors, and then finally, if required, levodopa (94). However, reductions in dopamine therapies can be challenging given the need to adequately treat motor symptoms. Importantly, psychoeducative approaches, including information about the nature of such symptoms, and more specific coping strategies may be helpful. Coping techniques include visual (looking in another direction or focusing more precisely on the hallucination), cognitive (turning light on, convincing oneself of the nonreality of the phenomenon), and interactive (speaking or interacting with a caregiver) approaches (95).
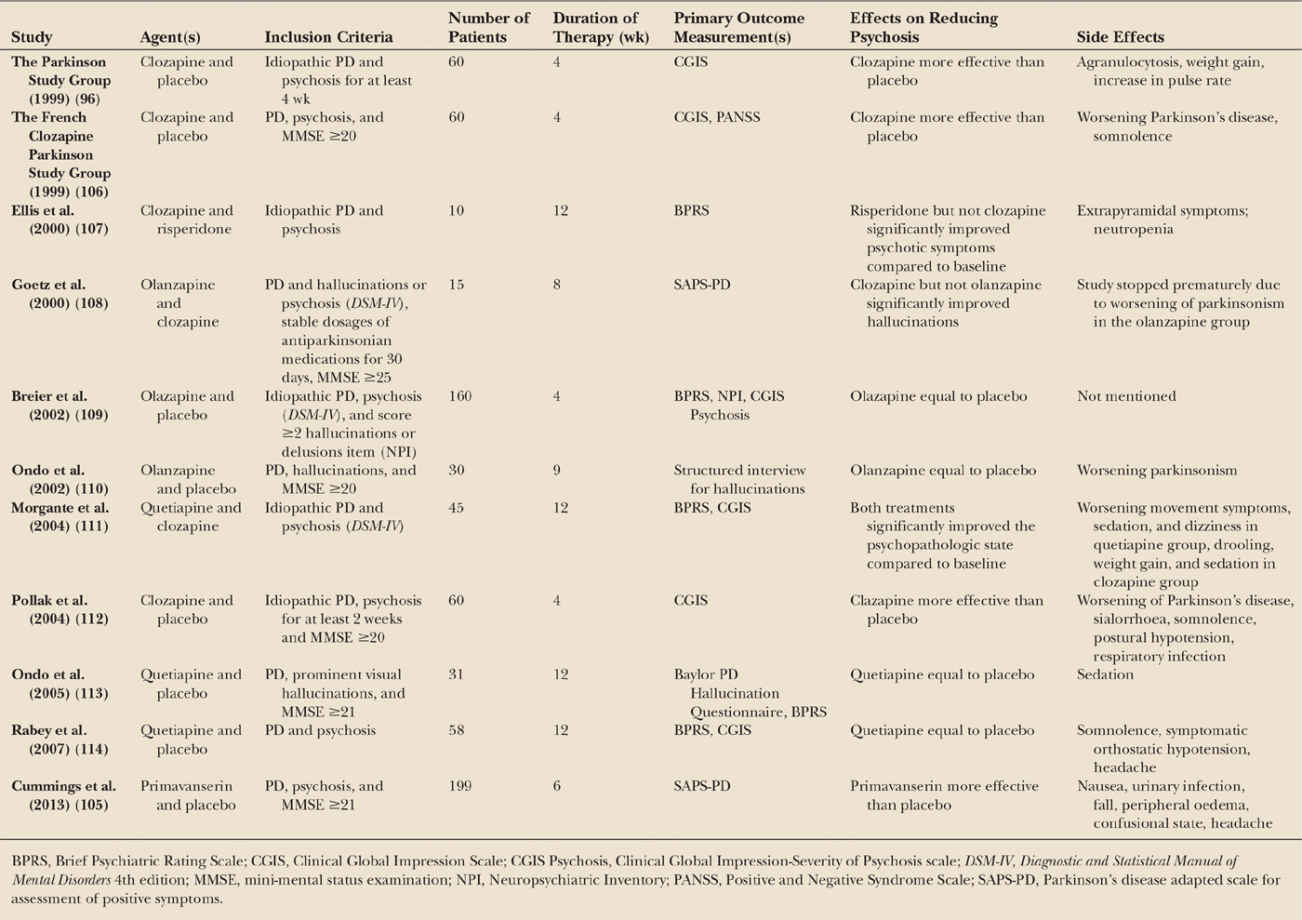
Specific pharmacologic interventions (Table 13.5) include the use of antipsychotics. The best evidence is for clozapine (96), which should be used in much lower dosages than used to treat schizophrenia, that is 12.5 to 50 mg/day. However, the widespread use of clozapine is limited due to its wide side effect profile, potential to induce agranulocytosis and the necessity for regular blood monitoring. As a result, other atypical antipsychotic agents are commonly used. However, both olanzapine and risperidone have demonstrated lack of efficacy and adverse events in PD patients and should not be used (97). Quetiapine is the antipsychotic used most frequently for PD psychosis, but there is little evidence from controlled studies for its efficacy in PD, with most showing no effect and/or important side effects. In a systematic review sponsored by the Movement Disorders Society, it was concluded that there is “insufficient evidence for the use of quetiapine for psychosis in PD,” but that the tolerability is acceptable (98). Of note, prolonged use of antipsychotics may have deleterious effects on cognition (99), and they significantly increase the risk of cerebrovascular events and mortality in older people with dementia in general (100). Therefore, although the available short-term studies suggested good tolerability, these agents should be used cautiously in PD patients, in particular in those with cognitive impairment.
ChEIs, in particular rivastigmine, may be an alternative first-line treatment option, as these agents can improve cognition, activities of daily functioning, and also NPS. The more frequent emergence of visual hallucinations as an adverse event in the placebo group in the EXPRESS study (101) suggests a potential protective effect against visual hallucination occurrence with rivastigmine in PDD. However, beyond small series, there is no systematic evidence that ChEIs can improve psychotic symptoms in PD.
Other drugs such as memantine (a NMDA antagonist) and ondansetron (a 5-HT3 antagonist) have also been considered for the treatment of PD psychosis. Overall, the therapeutic benefit of memantine remains inconclusive, and from a psychosis perspective improvements in the NPI in patients with PDD have not been observed, although there may be some benefit in DLB patients (102,103). Agitation may accompany psychosis in PD, in particular in dementia. Interestingly, a recent study showed that citalopram, an SSRI, improved agitation in AD (104), and may thus also have beneficial effects in people with PD, dementia, psychosis, and agitation.
A promising new drug for PD psychosis is pimavanserin, a selective serotonin 5-HT2A inverse agonist without dopaminergic, adrenergic, histaminergic, or muscarinic affinity. In a recent 6-week placebo-controlled trial, a significant benefit of pimavanserin on all endpoints, including the Scale for Assessment of Positive Symptoms in Parkinson’s Disease score was shown. Additional improvements were found in nighttime sleep and daytime somnolence (105). Overall pimavanserin was well tolerated and did not worsen motor symptoms. An interesting element of this study was that eligible participants entered a 2-week nonpharmacologic lead-in phase to limit the placebo response. Taken together, pimavanserin seems to be a viable alternative for the treatment of psychosis in PD.
DELUSIONAL MISIDENTIFICATION SYNDROME
The delusional misidentification syndrome (DMS) is an umbrella term for phenomena characterized by the misidentification or reduplication of persons, places, objects, or events (115). Christodoulou supposed that DMS consists of four basic syndrome subtypes, including Capgras syndrome, Fregoli syndrome, intermetamorphis, and subjective doubles (116). Other researchers included also misidentification of mirror image, misidentification of television, phantom boarder syndrome, reduplicative paramnesia, delusional hermaphroditism, and nurturing syndrome into DMS (117). Originally, DMS was thought to be associated only with psychiatric diseases; however, more recently DMS is also reported in neurologic and neurodegenerative diseases (117). In a prospective study, DMS was present in 16.7% of PD patients with dementia. The PD patients with DMS showed significant more impairment cognitive functioning, including language, semantic verbal fluency, and memory (118). As a consequence of the relatively high prevalence of DMS in neurodegenerative diseases, it is considered that DMS has an anatomical basis (117). Neuroimaging studies have shown lesions in the right frontal lobe and adjacent regions in patients with DMS. Medial temporal lobes structures may also be important (119). To date, no controlled pharmacologic treatment studies are performed. However, literature indicates that antipsychotics may be effective in patients with DMS (117).
| Double-Blind Controlled Studies of Pharmacologic Interventions for Psychosis in Parkinson’s Disease |