Chapter 2 Normal Human Sleep
An Overview
Sleep Definitions
According to a simple behavioral definition, sleep is a reversible behavioral state of perceptual disengagement from and unresponsiveness to the environment. It is also true that sleep is a complex amalgam of physiologic and behavioral processes. Sleep is typically (but not necessarily) accompanied by postural recumbence, behavioral quiescence, closed eyes, and all the other indicators one commonly associates with sleeping. In the unusual circumstance, other behaviors can occur during sleep. These behaviors can include sleepwalking, sleeptalking, teeth grinding, and other physical activities. Anomalies involving sleep processes also include intrusions of sleep—sleep itself, dream imagery, or muscle weakness—into wakefulness, for example (Box 2-1).
Box 2-1 Sleep Medicine Methodology and Nomenclature
In 2007, the American Academy of Sleep Medicine (AASM) published a new manual (see reference 50) for scoring sleep and associated events. This manual recommends alterations to recording methodology and terminology that the Academy will demand of clinical laboratories in the future. Although specification of arousal, cardiac, movement, and respiratory rules appear to be value added to the assessment of sleep-related events, the new rules, terminology, and technical specifications for recording and scoring sleep are not without controversy.
The current chapter uses the traditional terminology and definitions, upon which most descriptive and experimental research has been based since the 1960s.17 Hence, where the AASM terminology uses the term N for NREM sleep stages and R for REM sleep stages, N1 and N2 are used instead of stage 1 and stage 2; N3 is used to indicate the sum of stage 3 and stage 4 (often called slow-wave sleep in human literature); R is used to name REM sleep. Another change is to the nomenclature for the recording placements. Hence, calling the auricular placements M1 and M2 (rather than A1 and A2) is unnecessary and places the sleep EEG recording terminology outside the pale for EEG recording terminology in other disciplines. Although these are somewhat trivial changes, changes in nomenclature can result in confusion when attempting to compare to previous literature and established data sets and are of concern for clinicians and investigators who communicate with other fields.
Other issues are present in this new AASM approach to human sleep; however, this is not the venue for a complete description of such concerns. In summary, the AASM scoring manual has not yet become the universal standard for assessing human sleep and might not achieve that status in its current form. Specifications for recording and scoring sleep are not without controversy.51–56
NREM (pronounced “non-REM”) sleep is conventionally subdivided into four stages defined along one measurement axis, the electroencephalogram (EEG). The EEG pattern in NREM sleep is commonly described as synchronous, with such characteristic waveforms as sleep spindles, K-complexes, and high-voltage slow waves (Fig. 2-1). The four NREM stages (stages 1, 2, 3, and 4) roughly parallel a depth-of-sleep continuum, with arousal thresholds generally lowest in stage 1 and highest in stage 4 sleep. NREM sleep is usually associated with minimal or fragmentary mental activity. A shorthand definition of NREM sleep is a relatively inactive yet actively regulating brain in a movable body.
REM sleep, by contrast, is defined by EEG activation, muscle atonia, and episodic bursts of rapid eye movements. REM sleep usually is not divided into stages, although tonic and phasic types of REM sleep are occasionally distinguished for certain research purposes. The distinction of tonic versus phasic is based on short-lived events such as eye movements that tend to occur in clusters separated by episodes of relative quiescence. In cats, REM sleep phasic activity is epitomized by bursts of ponto-geniculo-occipital (PGO) waves, which are accompanied peripherally by rapid eye movements, twitching of distal muscles, middle ear muscle activity, and other phasic events that correspond to the phasic event markers easily measurable in human beings. As described in Chapter 141, PGO waves are not usually detectable in human beings. Thus, the most commonly used marker of REM sleep phasic activity in human beings is, of course, the bursts of rapid eye movements (Fig. 2-2); muscle twitches and cardiorespiratory irregularities often accompany the REM bursts. The mental activity of human REM sleep is associated with dreaming, based on vivid dream recall reported after approximately 80% of arousals from this state of sleep.1 Inhibition of spinal motor neurons by brainstem mechanisms mediates suppression of postural motor tonus in REM sleep. A shorthand definition of REM sleep, therefore, is an activated brain in a paralyzed body.
Sleep Onset
Definition of Sleep Onset
The precise definition of the onset of sleep has been a topic of debate, primarily because there is no single measure that is 100% clear-cut 100% of the time. For example, a change in EEG pattern is not always associated with a person’s perception of sleep, yet even when subjects report that they are still awake, clear behavioral changes can indicate the presence of sleep. To begin a consideration of this issue, let us examine the three basic polysomnographic measures of sleep and how they change with sleep onset. The electrode placements are described in Chapter 141.
Electromyogram
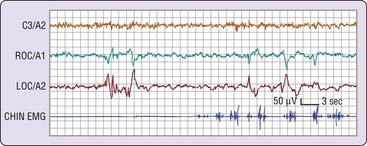
The electromyogram (EMG) may show a gradual diminution of muscle tonus as sleep approaches, but rarely does a discrete EMG change pinpoint sleep onset. Furthermore, the presleep level of the EMG, particularly if the person is relaxed, can be entirely indistinguishable from that of unequivocal sleep (Fig. 2-3).
Electrooculogram
As sleep approaches, the electrooculogram (EOG) shows slow, possibly asynchronous eye movements (see Fig. 2-3) that usually disappear within several minutes of the EEG changes described next. Occasionally, the onset of these slow eye movements coincides with a person’s perceived sleep onset; more often, subjects report that they are still awake.
Electroencephalogram
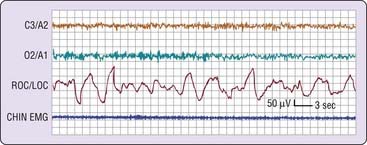
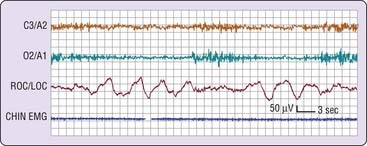
In the simplest circumstance (see Fig. 2-3), the EEG changes from a pattern of clear rhythmic alpha (8 to 13 cycles per second [cps]) activity, particularly in the occipital region, to a relatively low-voltage, mixed-frequency pattern (stage 1 sleep). This EEG change usually occurs seconds to minutes after the start of slow eye movements. With regard to introspection, the onset of a stage 1 EEG pattern may or may not coincide with perceived sleep onset. For this reason, a number of investigators require the presence of specific EEG patterns—the K-complex or sleep spindle (i.e., stage 2 sleep)—to acknowledge sleep onset. Even these stage 2 EEG patterns, however, are not unequivocally associated with perceived sleep.2 A further complication is that sleep onset often does not occur all at once; instead, there may be a wavering of vigilance before “unequivocal” sleep ensues (Fig. 2-4). Thus, it is difficult to accept a single variable as marking sleep onset. As Davis and colleagues3 wrote many years ago (p. 35):
Behavioral Concomitants of Sleep Onset
Given the changes in the EEG that accompany the onset of sleep, what are the behavioral correlates of the wake-to-sleep transition? The following material reviews a few common behavioral concomitants of sleep onset. Keep in mind that “different functions may be depressed in different sequence and to different degrees in different subjects and on different occasions” (p. 35).3
Simple Behavioral Task
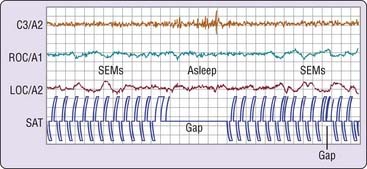
In the first example, volunteers were asked to tap two switches alternately at a steady pace. As shown in Figure 2-5, this simple behavior continues after the onset of slow eye movements and may persist for several seconds after the EEG changes to a stage 1 sleep pattern.4 The behavior then ceases, usually to recur only after the EEG reverts to a waking pattern. This is an example of what one may think of as the simplest kind of “automatic” behavior pattern. Because such simple behavior can persist past sleep onset and as one passes in and out of sleep, it might explain how impaired, drowsy drivers are able to continue down the highway.
Visual Response
A second example of behavioral change at sleep onset derives from an experiment in which a bright light is placed in front of the subject’s eyes, and the subject is asked to respond when a light flash is seen by pressing a sensitive microswitch taped to the hand.5 When the EEG pattern is stage 1 or stage 2 sleep, the response is absent more than 85% of the time. When volunteers are queried afterward, they report that they did not see the light flash, not that they saw the flash but the response was inhibited. This is one example of the perceptual disengagement from the environment that accompanies sleep onset.
Auditory Response
In another sensory domain, the response to sleep onset is examined with a series of tones played over earphones to a subject who is instructed to respond each time a tone is heard. One study of this phenomenon showed that reaction times became longer in proximity to the onset of stage 1 sleep, and responses were absent coincident with a change in EEG to unequivocal sleep.6 For responses in both visual and auditory modalities, the return of the response after its sleep-related disappearance typically requires the resumption of a waking EEG pattern.
Olfactory Response
When sleeping humans are tasked to respond when they smell something, the response depends in part on sleep state and in part on the particular odorant. In contrast to visual responses, one study showed that responses to graded strengths of peppermint (strong trigeminal stimulant usually perceived as pleasant) and pyridine (strong trigeminal stimulant usually perceived as extremely unpleasant) were well maintained during initial stage 1 sleep.7 As with other modalities, the response in other sleep stages was significantly poorer: Peppermint simply was not consciously smelled in stages 2 and 4 NREM sleep or in REM sleep; pyridine was never smelled in stage 4 sleep, and only occasionally in stage 2 NREM and in REM sleep.7 On the other hand, a tone successfully aroused the young adult participants in every stage. One conclusion of this report was that the olfactory system of humans is not a good sentinel system during sleep.
Response to Meaningful Stimuli
One should not infer from the preceding studies that the mind becomes an impenetrable barrier to sensory input at the onset of sleep. Indeed, one of the earliest modern studies of arousability during sleep showed that sleeping human beings were differentially responsive to auditory stimuli of graded intensity.8 Another way of illustrating sensory sensitivity is shown in experiments that have assessed discriminant responses during sleep to meaningful versus nonmeaningful stimuli, with meaning supplied in a number of ways and response usually measured as evoked K-complexes or arousal. The following are examples.
From these examples and others, it seems clear that sensory processing at some level does continue after the onset of sleep. Indeed, one study has shown with functional magnetic resonance imaging that regional brain activation occurs in response to stimuli during sleep and that different brain regions (middle temporal gyrus and bilateral orbitofrontal cortex) are activated in response to meaningful (person’s own name) versus nonmeaningful (beep) stimuli.11
Hypnic Myoclonia
What other behaviors accompany the onset of sleep? If you awaken and query someone shortly after the stage 1 sleep EEG pattern appears, the person usually reports the mental experience as one of losing a direct train of thought and of experiencing vague and fragmentary imagery, usually visual.12 Another fairly common sleep-onset experience is hypnic myoclonia, which is experienced as a general or localized muscle contraction very often associated with rather vivid visual imagery. Hypnic myoclonias are not pathologic events, although they tend to occur more commonly in association with stress or with unusual or irregular sleep schedules.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


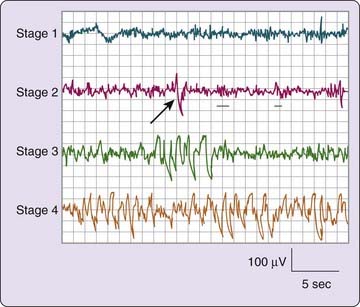
 -amplitude high-frequency setting of 30 Hz. On the second tracing, the arrow indicates a K-complex and the underlining shows two sleep spindles.
-amplitude high-frequency setting of 30 Hz. On the second tracing, the arrow indicates a K-complex and the underlining shows two sleep spindles.