Figure 75.1. [18F]FDG-PET (upper row left) showing normal glucose uptake. [18F]FCWAY-PET (top row right) shows decreased binding in the left temporal lobe, most pronounced in the amygdala and hippocampus. Lower row are axial views of [18F]FCWAY PET in a normal volunteer. There is no ligand binding in the cerebellum, reflecting absence of 5HT 1A receptors in cerebellar tissue. Raphe nucleus ligand binding can be seen. Left image is the right brain. (Courtesy of Dr. William H. Theodore. National Institutes of Health, Bethesda, Maryland.)
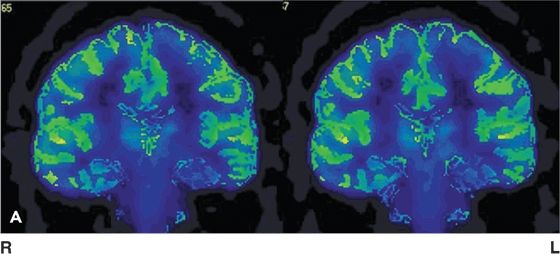
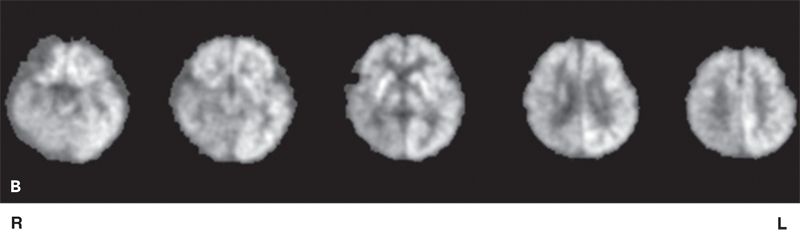
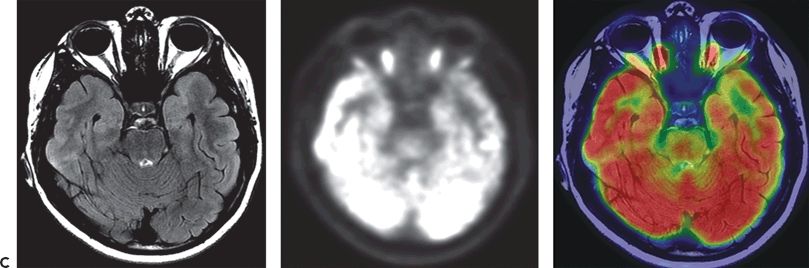
Figure 75.2. A: [18F]FDG-PET in an adult with left TLE showing decreased glucose uptake in mesial temporal regions following partial volume correction. (Courtesy of Dr. William H. Theodore. National Institutes of Health, Bethesda, Maryland.) B: [18F]FDG-PET scan in a 14-month-old child with focal seizures (right posterior quadrant focus), secondary generalization, and normal MRI. Figure shows right posterior quadrant hypometabolism. C: [18F]FDG-PET scan of a 15-year-old female, with focal epilepsy with altered consciousness, who has subtle left anterior mesial tip temporal focal cortical dysplasia (MRI) and widespread decrease in hypometabolism (PET). MRI, PET, and coregistered PET/MRI are shown. Left image is the right brain. (National Institutes of Health, Bethesda, Maryland.)
Sixty-five percent to 90% of patients with temporal lobe epilepsy (TLE) demonstrate regional hypometabolism; this figure is closer to 90% on recent generation scanners and approximately 60% for patients with normal MRI (4–6). The area of decreased glucose utilization is often more extensive than the epileptogenic zone and may extend into the adjacent inferior frontal or parietal lobe neocortex (3) and occasionally into the ipsilateral thalamus (7) and contralateral cerebellum (3). The regional abnormalities are invariably unilateral to the ictal focus. However, lobar localization is somewhat less reliable, about 80% to 90%. False lateralization as demonstrated after surgery (2) occurred in few patients, specifically when interpretation relied upon nonquantitative analysis, or occurred during subclinical seizures (2,8). Focal interictal regional hypometabolism also predicts a good surgical outcome (5,9–11). Different investigators using different methods and regional analyses have found several different regional hypometabolism patterns predictive of good outcome: inferior lateral temporal, anterior lateral, and uncus (5,9,11). Additionally, extent of resection of PET abnormalities correlates with postoperative outcome (12). Bilateral temporal hypometabolism is associated with a less optimistic surgical outcome and in half of patients reflects bilateral foci (13). Patients with focal temporal abnormalities have a 93% likelihood of good surgical outcome, and those without, 63% likelihood of good outcome (10,11). The ability to confirm the focus and predict surgical outcome is better when quantitative means are used, typically when asymmetry indexes [AI; e.g., AI = 2(left − right)/(left + right)] are greater than two standard deviations from normative data or about 10% to 15%. However, region of interest measures may be less useful for small, well-demarcated neocortical abnormalities that reflect limited focal cortical dysplasia. Lesser degrees of asymmetry, though visually apparent, may result in misleading information and erroneous conclusions (4,10,11). Voxel-based statistical methods performed in a standard anatomic atlas that allows comparison of individual patient images to normal control group data have been advocated as an alternative means of reliable analysis (14). Given that [18F]FDG-PET is often performed to confirm the focus, focal abnormalities may reduce the need for, or extent of, invasive monitoring when laterality of the focus is in doubt (2,10,11). Issues of frontal versus temporal focus may not always reliably be resolved by interictal [18F]FDG-PET studies, and invasive studies or other PET ligand studies may be needed. Conflicting localizing or lateralization data nearly always merit invasive monitoring.
Ictal [18F]FDG-PET studies are uncommon because of technical constraints such as ligand availability and unpredictability of seizures. They may show profound focal increases in glucose consumption, but results may also be normal or show decreased consumption. The results depend on the delivery of ligand, time and duration of the seizure, and degree of offsetting postictal hypometabolism. Although of interest, they are of limited clinical use, except during focal status epilepticus (15).
The reasons for regional hypometabolism are incompletely understood. Glucose consumption occurs primarily at the synapse. Regional hypometabolism appears to reflect a decrease in glucose influx from reduced glucose transport across the blood–brain barrier, which correlates with subsequent reduced phosphorylation. Cell loss with ensuing synaptic loss and altered remote projections, or degree of hippocampal atrophy in mesial temporal sclerosis, may account for a portion, but not all, of regional hypometabolism in TLE (16,17). Hypometabolism does not correlate with lifetime generalized tonic–clonic seizure or complex partial seizure (CPS) frequency (18). Dysplastic tissue with aberrant synaptic connectivity can have either decreased or normal glucose consumption (19). The abnormalities in some circumstances appear to be functional, as some patients have profound decreases in glucose uptake and no discernible pathology; regional decreased glucose uptake may vary with relation to previous ictal events and clinical manifestations of the previous seizure. In patients with mesial temporal sclerosis, the predominant regions that may manifest decreased glucose consumption are the lateral neocortex and, to a lesser extent, the frontal cortex. This may reflect the distant projection of functional loss in mesial structures. Frontal hypometabolism and contralateral hypometabolism appear to be reversible with successful temporal lobectomy (20).
Studies differ in the extent to which patients with mesial temporal seizures show pronounced lateral hypometabolism: mesial greater than lateral, lateral greater than mesial, and equal mesial and lateral temporal reductions in glucose uptake have been reported (3,4,21). Patients with neocortical temporal epilepsy may have greater lateral than mesial metabolic abnormalities (21). Patterns of hypometabolism may reflect seizure characteristics and seizure propagation. However, there is sufficient variability among patients that individual predictions of seizure focus within the temporal lobe based on [18F]FDG-PET cannot be made.
[18F]FDG-PET will be abnormal when MRI shows significant abnormalities, for example, in mesial temporal sclerosis, tumor, vascular malformation, infarct, and most instances of cortical dysplasia. In this setting, [18F]FDG-PET provides little additional information beyond MRI. [18F]FDG-PET may be more sensitive than MRI in TLE under some circumstances. Current PET techniques are helpful in 85% to 90% of patients, volumetric MRI in 60% to 70%, and magnetic resonance spectroscopy (MRS) in 55%. Higher-resolution scanning techniques, including high-resolution fast spin echo, fluid-attenuated inversion recovery, T2 relaxometry, magnetization transfer, and high-resolution thin-cut spoiled gradient recall anatomic sequences, and use of higher magnetic field strength (3T and now 7T) have reduced the utility of [18F]FDG-PET (22). Comparison studies report varying efficacy results with different imaging modalities, which frequently reflect the particular research strengths of the investigators rather than the intrinsic advantages of the techniques studied.
Although glucose consumption in the temporal cortex is decreased, perfusion is often maintained, especially in the lateral neocortex (4,23). Interictal studies of cerebral blood flow using [15O]water find a decrease in perfusion in only 50% of patients, but one-fifth of these provide falsely localizing information (4). This experience is similar to that in interictal SPECT studies and quantitative perfusion ascertained by arterial spin-labeled fMRI (5,24). These data suggest that vascular tone may be impaired in TLE and that the relationship between metabolism and perfusion is altered. For these reasons, interictal blood flow studies are unreliable markers of the epileptogenic zone and do not predict surgical outcome (4).
FDG-PET in Newly Diagnosed and Nonrefractory Localization–Related Epilepsy
Metabolic abnormalities are less common in patients with recent-onset, nonrefractory, or well-controlled partial epilepsy. Thirty percent of adults with nonlesional epilepsy within <3 years of seizure onset have focal [18F]FDG-PET (25). Forty to 50 percent of adults without refractory seizures of limited duration (<5 years) have focal abnormalities (25,26). Presence of focal abnormalities does not predict 2-year outcome. Other studies report 20% of adults with well-controlled partial seizures had regional metabolic abnormalities. In these adult populations, localization of seizures is less certain than in patients with refractory epilepsy—an important consideration because patients with extratemporal lobe epilepsy are less likely to have abnormal [18F]FDG-PET studies.
Chronic partial epilepsy typically begins during childhood. Twenty percent of children with new-onset focal epilepsy and normal MRI demonstrate regional hypometabolism. All (ipsilateral) abnormalities occurred among the children with a suspected temporal lobe focus. (27). Follow-up studies did not show any change in extent or magnitude of regional hypometabolism; a combination of MRI and PET findings predicted outcome—those with persistent abnormalities fared less well (28). In another study, regional hypometabolism changed in relation to seizure frequency in children with worsening seizures (29). Similar to adults, 70% of children with chronic partial epilepsy (duration 10 years) have focal metabolic abnormalities. There is evidence that adult patients with a greater duration of epilepsy are more likely to have focal [18F]FDG-PET abnormalities (3,23,27). Partial seizures of greater duration are also associated with a greater dissociation between metabolism and blood flow (4,23). These [18F]FDG and cerebral blood flow studies, along with cross-sectional studies using volumetric MRI, may be taken as evidence that TLE in some patients is associated with chronic and continued neuronal injury (23,30).
Other PET Ligands in Temporal Lobe Epilepsy
In addition to widespread reduction of glucose utilization in cortical projection areas, with relatively preserved perfusion, ligand-binding studies reveal additional functional abnormalities in patients with TLE (Table 75.1). These findings reflect hippocampal atrophy, loss of neuron populations, or a neuronal response to epilepsy.
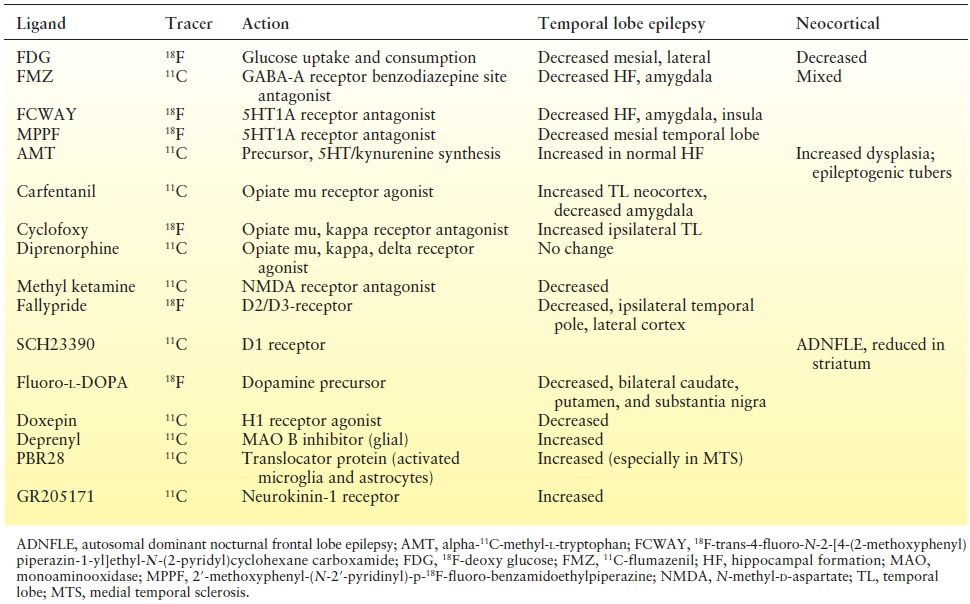
Table 75.1 PET Ligands in Temporal and Neocortical (Nonlesional) Epilepsy
GABA-A Receptor Studies
Unlike [18F]FDG-PET, which typically demonstrates hypometabolism that is more widespread than the epileptogenic zone, PET with [11C]flumazenil ([11C]FMZ), a benzodiazepine antagonist of the gamma-aminobutyric acid (GABA)-A receptor, shows focal abnormalities confined to the hippocampal formation (6,31). Autoradiography of pathologic tissue indicates that most decreased [11C]FMZ binding is proportional to cell loss (6). In contrast, some [11C]FMZ-binding studies performed in patients with mesial temporal sclerosis argue for an absence or downregulation of GABA receptors beyond that expected by atrophy alone. After accounting for partial volume effect, a 38% reduction in [11C]FMZ binding is found in sclerotic hippocampus beyond reduction in hippocampal formation volume (31). In partial epilepsy, a greater degree and extent of decreased [11C]FMZ binding are seen in patients with more frequent seizures, and decreased binding may extend to projection areas of the epileptogenic region (32). In mesial temporal sclerosis, there is decreased [11C]FMZ binding in one-third of patients in the contralateral hippocampal formation but to a lesser extent than in the epileptogenic hippocampus. This finding is similar to those in MRS studies. However, in patients with a temporal focus and normal MRI, [11C]FMZ-PET is less useful (6). SPECT with [123I]IMZ, a benzodiazepine ligand (33), shows results similar to those of the PET ligand and more useful than cerebral blood flow–based SPECT (34).
Serotonin Receptor and Synthesis Studies
Serotonin (5HT) IA receptor binding is reduced, to a greater degree than reduced glucose uptake, in epileptogenic mesial temporal lobe and adjacent insula as deduced by the selective antagonists including those with normal MRI [18F]trans-4-fluoro-N-2-[4-(2-methoxyphenyl)piperazin-1-yl]ethyl]-N-(2-pyridyl)cyclohexanecarboxamide ([18F]FCWAY) and [18F]2′-methoxyphenyl-(N-2′-pyridinyl)-p-18F-fluoro-benzamidoethylpiperazine [18F]MPPF (see Fig. 75.1) (35–37). Regional [18F]FCWAY binding contributes to likelihood of achieving seizure freedom following temporal lobe resection (10). Alpha-[11C]methyl-l-tryptophan ([11C]AMT) is increased in the hippocampus ipsilateral to mesial TLE in patients with normal hippocampal formation volumes, but not MTS (38). [11C]AMT, designed as a serotonin precursor, may also be a marker for quinolinic or kynurenic acid, compounds implicated in excitatory neurotransmission (38,39). Decreased receptor binding in mesial temporal structures is more pronounced than reductions in cerebral metabolism. Decreased binding also correlates with severity of depression in epilepsy patients (40) comparable to patients with primary depression.
Other Ligands
Table 75.1 lists findings from several studies of investigational ligands in limited populations using probes for opiate, histamine, and NMDA receptors as well as glial markers. Studies using [11C] PBR28, a probe of the translocator protein that is increased in activated microglia and astrocytes, hence a marker of inflammation, find increased binding in the hippocampus, amygdala, and fusiform gyrus in the epileptogenic temporal lobe; the asymmetry was highest in patients with mesial temporal sclerosis (Fig. 75.3) (41).
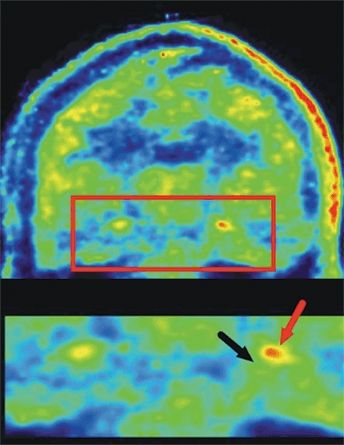
Figure 75.3. Coronal image from [11C] PBR28 PET in an adult patient with left-sided TLE demonstrating increased ligand uptake in the epileptogenic hippocampus (black arrow hippocampus; red arrow increase ligand binding in hippocampal formation): the hippocampal area is magnified in the bottom row. Left image is the right brain. (Courtesy of Dr. William H Theodore, National Institutes of Health, Bethesda, Maryland;. Hirvonen J, et al. Increased in vivo expression of an inflammatory marker in TLE. J Nucl Med. 2012;53(2):234–240.)
PET in Extratemporal Lobe and Neocortical Epilepsy
[18F]FDG-PET is less efficacious in identifying the epileptogenic zone in extratemporal lobe epilepsy than in TLE (42). Most extratemporal lobe epilepsy series include patients with structural lesions that, not surprisingly, show concordant hypometabolism. When patients with abnormal MRI findings are excluded, 11% to 50% of the relatively small patient populations remaining show regional decreases in glucose consumption (6,43). Some investigators have found a good correlation between regional hypometabolism and the epileptogenic zone; others have found a reasonable correlation with side, but not site, of ictal origin. FDG-PET abnormalities remote from the lesion lessen prospects of surgical outcomes. Coregistration of FDG-PET and high-resolution MRI may increase yield of identifying malformations of cortical development, the most common presumed cause of “nonlesional” epilepsy in children and adults (see Fig. 75.2C) (44,45).
[11C]FMZ-PET studies yield mixed and inconsistent results (6,46). [11C]FMZ binding may be reduced and is more restricted in cortical extent than [18F]FDG-PET abnormalities, when present. It appears to correlate with the site of ictal activity and, if resected, is associated with improved outcome (47). Patients with acquired lesions may have regional focal reductions in [11C]FMZ binding concordant with the lesion, but most marked at the margins (46). In other studies, two-thirds of patients with neocortical epilepsy and normal MRI had [11C]FMZ abnormalities, either increased or decreased, which were bilateral in half of the subjects (46,48). Techniques that correct for gray matter volume averaging may be helpful in identifying abnormal [11C]FMZ binding in cortical dysplasia (48). Given these mixed findings, the role of [11C]FMZ in nonlesional epilepsy remains unclear. In patients with extratemporal lobe partial epilepsy, ictal SPECT may be a better identifier of the epileptogenic cortex, which is discussed below.
PET in Generalized Epilepsy
PET has been used to explore generalized epilepsies, predominantly of the absence type. Glucose consumption and perfusion are globally increased (49). [15O]Water studies performed during electroencephalographic (EEG) bursts of spike and wave demonstrate not only an increase in global perfusion but also a preferential increase in the thalamic regions, supporting the notion of the thalamus as the facilitator of absence events (50). There are no differences in [11C]FMZ or [11C]diprenorphine binding in absence epilepsy. In juvenile myoclonic epilepsy, [(11)C]PE2I, a marker of dopamine transporter activity, is reduced in the midbrain and the high-affinity dopamine (D2/D3) receptor ligand [18F]fallypride ([(18) F]FP) is reduced in the putamen (51).
PET in Children with Epilepsy
[18F]FDG-PET studies of normal development show increased glucose utilization in all brain areas, peaking around 5 to 8 years of age, that parallels synaptic (52). Mature patterns of glucose uptake are established in the primary motor and sensory cortex before they are consolidated in the association cortex. [18F]FDG-PET studies of children with partial epilepsy show regional abnormalities similar to those seen in adults with temporal or extratemporal lobe epilepsy and are discussed above (see Fig. 75.2). Although primary generalized epilepsies are typically viewed as pediatric disorders, imaging studies in these populations have only been performed in adults (see PET in Generalized Epilepsy). Pediatric epilepsy syndromes that have been studied include infantile spasms, Lennox–Gastaut syndrome, Landau–Kleffner syndrome, Rasmussen encephalitis, and several of the cortical dysplasias, including tuberous sclerosis.
Children with infantile spasms may show extensive hypometabolism, usually in posterior brain regions (19). These abnormalities often correspond to MRI abnormalities and may identify areas of dysgenesis not readily apparent with older MRI techniques. However, some children with a generalized EEG and normal MRI exhibit regional metabolic abnormalities (53). PET has been used in these cases to remove the epileptogenic zone in children with catastrophic epilepsy. In some children, however, the metabolic abnormalities seen at onset of infantile spasms may resolve with time and thus may represent a functional state that is potentially reversible with successful medical therapy (54). In children with Rasmussen encephalitis and hemimegalencephaly, widespread hemispheric hypometabolism is typically seen. PET has been advocated in some circumstances to assess the integrity of the good hemisphere before extensive cortical resection (19,55).
In tuberous sclerosis, tubers are often hypometabolic, whereas there is some evidence that the more epileptogenic tubers have increased serotonin or kynurenic acid synthesis, reflected by increased [11C]AMT uptake (56). [11C]AMT uptake is also increased in focal cortical dysplasia; MRI (especially in children <2 years) and [18F]FDG-PET may be normal (Fig. 75.4) (39,56).
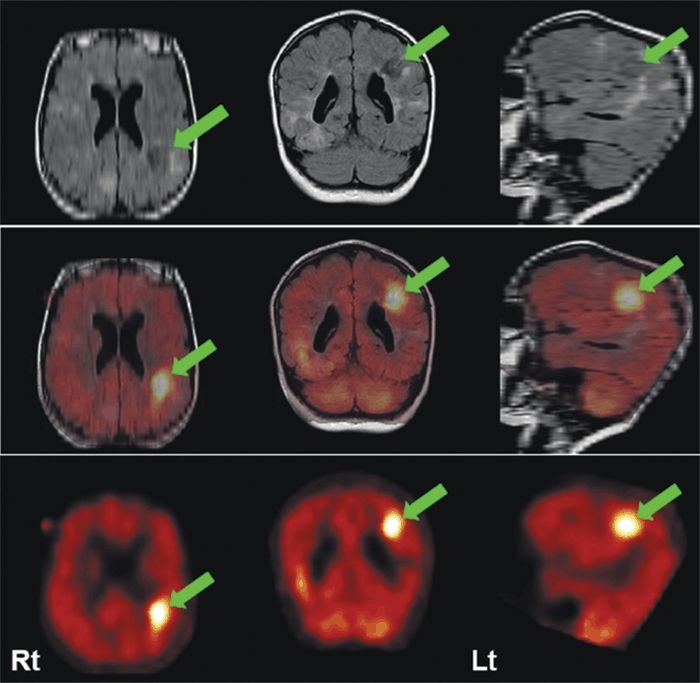
Figure 75.4. MRI (FLAIR) (upper row) and [11C] AMT-PET (bottom row) and coregistered images (middle row) in a 2-year-old child with tuberous sclerosis. [11C] AMT-PET shows markedly increased ligand uptake in epileptogenic parietal tuber (arrow) that is hypometabolic with [18F]FDG-PET (not shown). (Courtesy of Dr. Harry Chugani, Detroit Children’s Hospital.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








