In 2004, Defrin and coworkers [30] measured heat-pain threshold in 25 individuals with ID, 11 of which had Down’s syndrome and 14 with nonspecified ID. Pain threshold in the forearms was measured using a computerized thermal stimulator with two different methods; the method of limits, which includes a reaction-time artifact (as subjects are required to press a switch upon pain detection, thereby ceasing the stimulus), and the reaction-time-free method of levels (as subjects report postfactum whether a predetermined stimulus intensity was painful or not). The authors found that individuals with ID had a similar pain threshold to that of controls when measured with the method of limits; however, they had a significantly lower pain threshold compared to controls when measured with the method of levels (see Fig. 8-1).
The measurement of heat-pain threshold with reaction time–dependent and –independent methods enabled the calculation of the reaction time and nerve conduction velocity of the participants [30]. Reaction time was found to be significantly slower in individuals with ID compared with controls and nerve conduction velocity (compatible with that of C fibers) tended to be slower, more so in individuals with Down’s syndrome compared with controls (see Fig. 8-2). Since reaction time is intrinsic to the method of limits, slow reaction time induces an artificial elevation of the pain threshold. Thus, the lack of elevation in heat-pain threshold in individuals with ID despite slower reaction time using the method of limits and the decreased heat-pain threshold measured with the method of levels implies that individuals with ID indeed have lower pain threshold compared to normal. The results of this study suggested that contrary to previous belief, individuals with MR are even more sensitive to pain than the normal population; however, they may seem less sensitive to pain due to slow reactions. These authors thus proposed that measurements of pain threshold in these individuals should be conducted using methods that do not rely on reaction time.
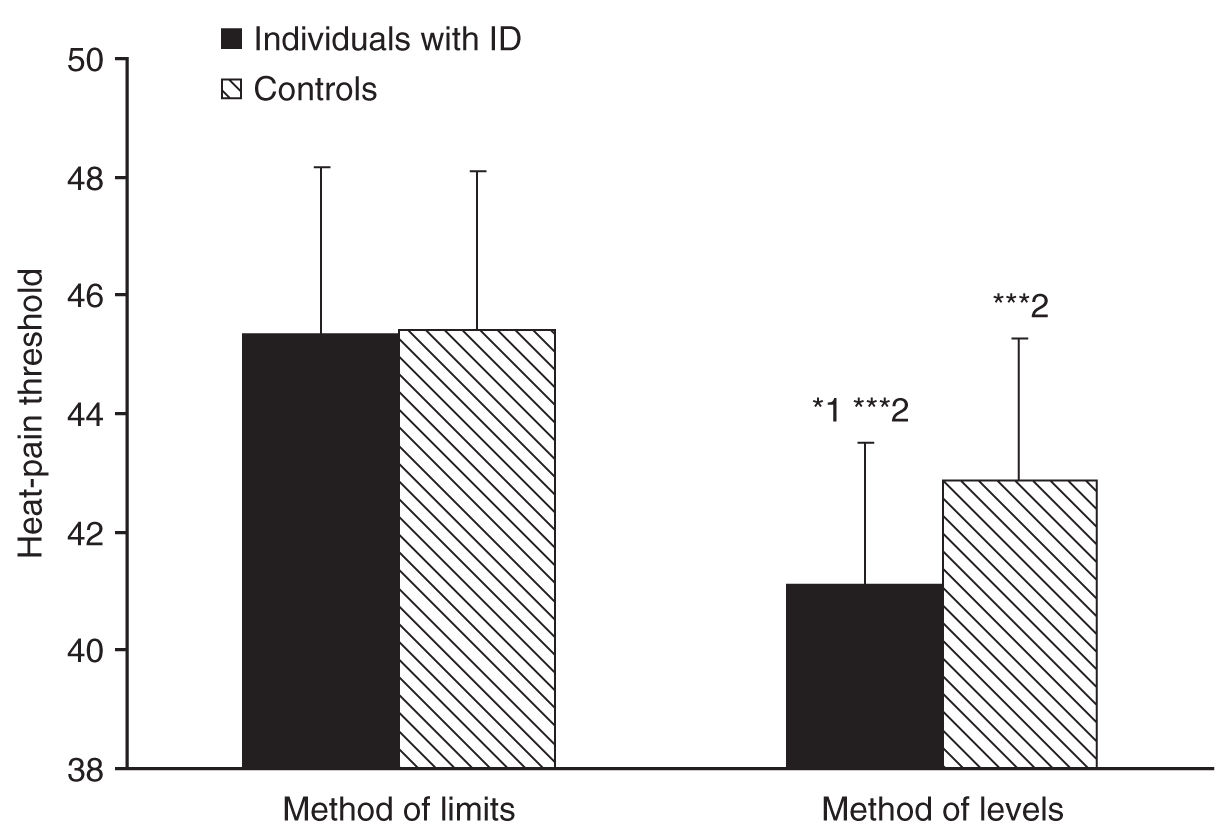
FIGURE 8-1 Individuals with intellectual disability (ID) had similar heat-pain threshold as controls when measured with the reaction-time-dependent method of limits. However, individuals with ID had significantly lower heat-pain threshold compared to controls when measured with the reaction-time-free method of levels (*1, P < 0.05). In both groups, heat-pain thresholds measured with the method of levels were significantly lower than those measured with the method of limits (***2, P < 0.0001). Bars denote group means ± SD.
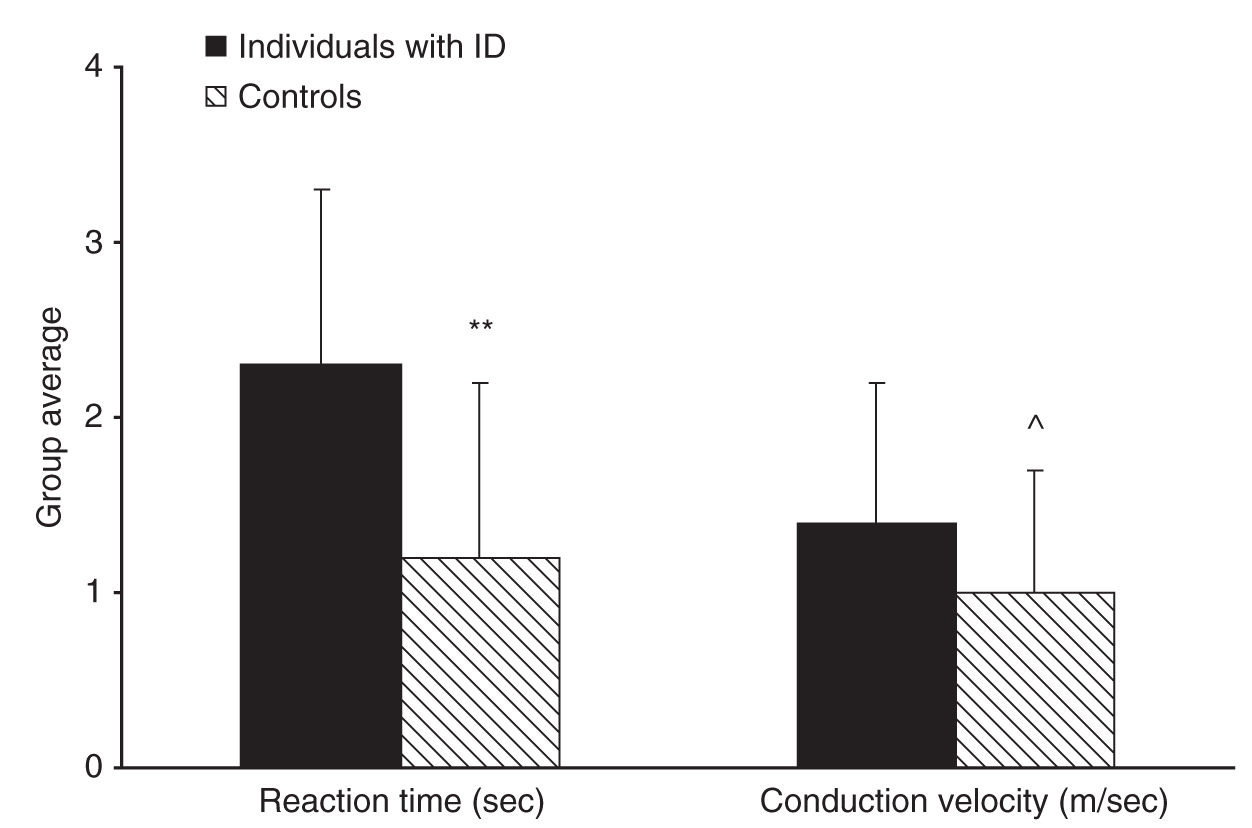
FIGURE 8-2 Individuals with intellectual disability (ID) had significantly slower reaction time than controls (**P < 0.01) and tended to have slower nerve conduction velocity than controls (^P = 0.064) as calculated from the two pain threshold measurements. Bars denote group mean ± SD.
Cascio et al. [16] measured heat- and cold-pain thresholds with computerized thermal stimulator among eight adults with high functioning autism (either autistic disorder or Asperger disorder). The authors used the method of limits and found that pain thresholds were significantly lower than in matched control. Priano et al. [76] too measured heat- and cold-pain thresholds using computerized thermal stimulator with the method of limits in 14 adolescents with Prader–Willi syndrome, a neurogenetic developmental disorder with a tendency to self-injury. The authors found that the thresholds of the participants, measured in the hands were increased compared to controls and concluded that these individuals are hyposensitive to pain. The authors also found that the latencies and conduction velocities of the median and ulnar nerves subserving the hand are slower than those of controls; however, their measurements reflected the large myelinated fibers not involved in heat and cold conduction. Therefore, it was not clear whether the increased pain thresholds were due to the slower nerve conduction velocity.
Riquelme and Montoya [81] recently found that children (but not adults) with cerebral palsy had increased sensitivity to pressure pain in the lips than controls when measured with a modified method of limits. Noteworthy, however, is that out of 29 participants, only 15 had ID and their contribution to the results was not examined.
Peripheral and central nerve conduction in ID was evaluated in only a few more studies with inconsistent results. To the best of our knowledge, all the electrophysiological recordings in this population were done with innocuous stimuli, and therefore, the results may not be applied to the pain system. For example, as in the study of Priano et al. [76], slower median nerve conduction velocities and lower nerve action potential amplitudes compared to controls were also found in five children with Down’s syndrome [5] but not in five children with Prader–Willi syndrome [6]. The latter exhibited normal somatosensory-evoked potentials measured in the scalp as in Priano et al. [76] but also lower than normal amplitudes, as in the case of children with cerebral palsy with and without ID [81]. Normal median nerve conduction velocity was also seen in two patients of brothers with ID suffering from hereditary [74] and congenital [50] insensitivity to pain associated with self-injury; however, nerve biopsies from these two patients revealed low numbers of myelinated and unmyelinated nerve fibers. Prolonged latencies of somatosensory-evoked potentials as well as of brain stem auditory-evoked potentials and visual-evoked potentials found in infants with Down’s syndrome [18] may suggest that they may suffer from various sensory deficits including the nerve conduction of noxious stimuli. The above-mentioned findings suggest that myelinization of sensory nerve fibers and the number of normal nerve fibers may be affected in some but not all ID syndromes. Further studies are needed to determine whether alterations exist also in nerve conduction of noxious stimuli.
In the absence of additional studies, in which pain threshold was directly measured, and on the basis of the electrophysiological findings, we may conclude that individuals with ID especially those with Down’s syndrome or otherwise non-specified ID are certainly not less sensitive to pain than normal and may even be more sensitive to pain than normal. The inconsistent evidence with regard to pain threshold among the different disability types (see Table 8-1) could reflect differences in pain processing between the syndromes. We may also conclude that alterations in peripheral and/or central nerve conduction of sensory signals may be responsible for the delayed responses seen in methods that include reaction time. Due to the possibility that individuals with developmental disability exhibit alterations in nerve conduction, measuring pain threshold with methods that bypass this limitation, that is, reaction-time-free methods are preferable.
PAIN SCALING ABILITIES OF INDIVIDUALS WITH DEVELOPMENTAL DISABILITY
Ability to self-report is the basis not only for threshold measurements but also for freely communicating pain during interviews. Interviewing individuals with developmental disability is important; although depending on their cognitive impairment, they sometimes are still the best source of information regarding their health. The results of studies are inconsistent with regard to the ability of individuals with developmental disability to use pain scales. For example, Dagnan and Ruddick [27] found that 25 out of 29 individuals with mild and moderate ID were able to give reliable responses using visual analog scale consisting of two pictorial anchors (one smiling and one crying) with a 5-inch line between them. Validity of self-report was also obtained using colored analog scale in children and adults with mild ID [41, 60]. Furthermore, adults with mild to moderate ID were found to reliably use the 21-point (0–100) box scale to rate their chronic pain over time [19]. The scale has a row of 21 boxes labeled from 0 to 100, in increments of five. The 0 anchor is labeled “no pain,” while the 100 anchor is labeled “pain as bad as it could be.” To complete the scale, respondents indicate the box that best represents their pain. It is noteworthy that reliability was inversely correlated with the ID level, with those less affected being more reliable in their ratings.
In contrast, LaChapelle et al. [49] found that 35% of individuals with severe ID were not able to respond to a simple question on their pain intensity in response to an injection, and those who responded provided an unreliable pain rating. Furthermore, their pain reports did not correspond with their facial expression during injection [49]. Similarly, Defrin et al. [29] found that adults with various levels of ID could not reliably use a five-faces scale to report their pain. These individuals choose either the smiling face (which indicates no pain) or the face in the middle of the scale, which has no particular expression regardless of whether they were at rest or subject to needle stick. Here again, their pain reports using the faces scale did not correspond with their facial expression or their bodily movements during injection [29]. In another study, 60% of children with cerebral palsy failed a validity test designed to assess their understanding of a faces pain scale [52]. It is possible that these subjects simply cannot use faces scale because they do not comprehend the meaning of ranks and cannot dispose their pain experience onto faces. Furthermore, it has been postulated that short scales (2–6 points) have an increased risk of scale attenuation effect, resulting in reduced rating variability and discrimination [19]. Other, graphical or three-dimensional, tangible scales might be more suitable. The poker chip tool is a good example. The tool was found suitable for small children as well consists of four chips that represent “pieces of hurt”, with one chip indicating a “little hurt” and all four chips indicating “the most hurt a person could have.”
In summary, it appears that not all individuals with developmental disability are able to use scales to report pain; nor is every scale suitable for every individual. Further study is needed to explore the best rating scale for different levels of developmental disability. Nevertheless, the inability to use pain rating scales does not necessarily preclude the ability to provide free verbal report on the existence of pain or grossly quantify its intensity, which has considerable clinical significance.
BEHAVIORAL INDICES OF PAIN IN INDIVIDUALS WITH DEVELOPMENTAL DISABILITIES
Although self-report is considered a gold standard of pain assessment, pain threshold and direct scaling cannot be applied to individuals who have a limited verbal ability to report pain. In these instances other methods that do not rely on direct communication should be applied. Facial activity is perhaps the most immediate and intuitive sources of information available, and hence the analysis of facial expressions of pain is the most common way to study pain among noncommunicative individuals with and without ID. A specific method termed facial action coding system (FACS) was developed by Ekman and Friesen [35], on the premise that the face displays considerable plasticity and expresses stereotypical reactions to different situations including pain. The movements of a single or a group of muscles in the face called “action units” (AUs) are coded, and the frequency or intensity of these AUs are quantified. A substantial body of work has been published, mostly by Craig and colleagues on the validity and reliability of the FACS as a measurement tool for pain in newborns and children and later in elderly people with dementia [26, 42, 48, 51, 88].
In addition to facial expressions, body gestures, vocalization, and other behavioral expressions have been coded among individuals with developmental disability, using various tools. One example is the noncommunicating children’s pain checklist (NCCPC) developed by McGrath and coworkers [12, 62]. The NCCPC allows the observer to score the frequency of occurrence of behaviors comprising the following categories: vocal, eating, sleeping, social, facial, activity, body and limb, and physiological signs. Additional tools include, but are not restricted to, the pain and discomfort scale (PADS) [3] and the face legs activity cry and consolability (FLACC) behavioral pain assessment tool [55].
Facial expressions among individuals with ID were analyzed during acute and chronic pain conditions. Facial expressions during acute pain are usually analyzed before and during a medical procedure deemed painful such as needle stick associated with vaccination or blood draw and dental cleaning. In several studies, the overall facial activity of individuals with ID was increased compared to their baseline level [12, 29, 34, 42, 49, 75, 98]. Most of these studies lacked a control group of cognitively intact individuals, and therefore, it is not clear whether the increase in facial expression was different than normal. The two studies that had such a control group revealed that children [34] or adults [29] with mild–moderate ID displayed changes in pain behavior similar to those of controls, whereas children who were unable to verbalize their pain [34] or adults with severe-profound ID [29] exhibited elevated or atypical facial expressions at baseline compared with controls that affected the magnitude of the change in pain behavior. Expressions such as closing and squinting of eyes, grimacing, brow furrowing, and mouth opening are common in these individuals during pain. However, other facial expressions that are not intuitively associated with pain also appear during acute painful conditions. These may include moving the eyes from side to side, tongue protrusion, and a smile [29].
Facial reactions to pain were also examined in children with ASD. Here again, facial expressions were increased during acute pain compared to baseline and to a greater extent and longer duration after the end of the venipuncture compared with controls [68, 79, 95]. Furthermore, individuals with ASD displayed a significantly increased heart rate in response to venipuncture that was significantly greater than in controls [95]. These findings contradict the commonly held view on insensitivity to pain among individuals with ASD. One study did not find an increase in facial expression of adolescents with cerebral palsy during vaccination compared to sham control; however, the subjects were reported to suffer from chronic pain which might have affected their response [70]. It may thus be concluded that prior reports of reduced pain sensitivity in ID and ASD are related to a different mode of pain expression rather than to insensitivity to pain.
In the minority of facial expression studies on individuals with ID, experimental rather than clinical stimuli were used [87, 90]. These authors administered four innocuous (warm, cold, pressure, and light touch) and one presumably noxious (pin prick) stimulation modalities to 44 adults with severe and profound of ID. The authors found increased facial activity during the application of all the stimuli compared to baseline but did not find differences in facial activity between the different stimulation modalities.
As with facial expression, general bodily movements were also increased in individuals with ID and ASD during acute painful medical procedures compared to baseline [29, 34, 75, 79, 91]. Here again, individuals with more severe ID had more pronounced or atypical body gestures than those with milder ID. In one study, the increase in bodily movements was more pronounced among individuals with self-injurious behavior as compared to those without self-injurious behavior [91]. It should be pointed out that as with facial expression, behaviors that are not intuitively associated with pain might appear. For example, Defrin et al. [29
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






