Parkinson Disease
Bradley J. Robottom
Lisa M. Shulman,
William J. Weiner
key points
Parkinson disease is the second most common neurodegenerative disease worldwide.
Parkinson disease has four major cardinal signs: resting tremor, cogwheel rigidity, bradykinesia, and postural instability.
Resting tremor is the most common presenting symptom.
Nonmotor symptoms such as depression, apathy, and anxiety are common and have a negative impact on quality of life.
A variety of effective, symptomatic treatments are available.
Antipsychotics and other dopamine blocking agents should be avoided in Parkinson disease patients.
A careful medication history asking about exposure to dopamine blocking agents is important in making a diagnosis of Parkinson disease.
Parkinson disease is the most common akinetic rigid syndrome and the most frequently encountered extrapyramidal movement disorder. It is a neurodegenerative disease of unknown etiology that most often begins at 58 to 60 years of age. Approximately 10% to 15% of patients will have disease onset before age 50. As the population of the United States ages, the number of people at risk for the development of Parkinson disease increases. Diagnostic and therapeutic knowledge is important not only because of the prevalence of the disorder but also because the pharmacology of Parkinson disease has led to fundamental changes in the way investigators and physicians view central nervous system neurotransmitter function.
CLINICAL FEATURES
Parkinson disease is characterized by a typical history of progressive neurologic disability and the following four major neurologic signs: resting
tremor, cogwheel rigidity, bradykinesia, and impaired postural reflexes. It is often observed that when a patient first presents to a physician for the evaluation of symptoms, the syndrome has been present for 1 to 2 years. Unilateral tremor involving a single limb is the most common presenting symptom and sign. However, careful history taking often reveals that difficulty buttoning shirts or blouses, fastening snaps, cutting food; alterations in handwriting (Fig. 12.1); a feeling of stiffness; or a feeling of overall slowness may have been noted up to 12 to 24 months earlier and that these symptoms have gradually become worse. In addition, a patient may note voice fluctuations and intermittent loss of volume. Inquiring whether the patient has difficulty rising from low, soft chairs or sofas; difficulty entering and leaving an automobile, difficulty turning in bed; or difficulty walking and maintaining balance in a crowd highlight the functional impact of bradykinesia, rigidity, gait impairment, and impaired postural reflexes. The patient may occasionally notice an inability to stop walking forward (propulsion) or backward (retropulsion). Family members may also report that the patient’s facial expression has changed and that he or she does not smile as much (masked faces; Fig. 12.2), that he or she seems to stare all the time (reptilian stare), that her or his posture has become stooped and flexed (simian posture; Fig. 12.3), and that he or she has become exasperatingly slow. It may take 30 to 90 minutes to dress in the morning and even longer to disrobe in the evening.
tremor, cogwheel rigidity, bradykinesia, and impaired postural reflexes. It is often observed that when a patient first presents to a physician for the evaluation of symptoms, the syndrome has been present for 1 to 2 years. Unilateral tremor involving a single limb is the most common presenting symptom and sign. However, careful history taking often reveals that difficulty buttoning shirts or blouses, fastening snaps, cutting food; alterations in handwriting (Fig. 12.1); a feeling of stiffness; or a feeling of overall slowness may have been noted up to 12 to 24 months earlier and that these symptoms have gradually become worse. In addition, a patient may note voice fluctuations and intermittent loss of volume. Inquiring whether the patient has difficulty rising from low, soft chairs or sofas; difficulty entering and leaving an automobile, difficulty turning in bed; or difficulty walking and maintaining balance in a crowd highlight the functional impact of bradykinesia, rigidity, gait impairment, and impaired postural reflexes. The patient may occasionally notice an inability to stop walking forward (propulsion) or backward (retropulsion). Family members may also report that the patient’s facial expression has changed and that he or she does not smile as much (masked faces; Fig. 12.2), that he or she seems to stare all the time (reptilian stare), that her or his posture has become stooped and flexed (simian posture; Fig. 12.3), and that he or she has become exasperatingly slow. It may take 30 to 90 minutes to dress in the morning and even longer to disrobe in the evening.
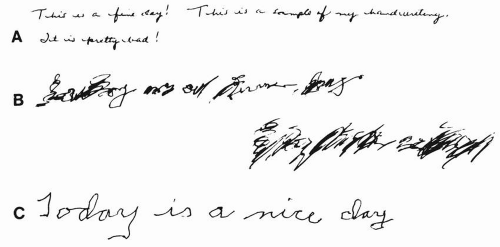 FIGURE 12.1 A: This handwriting sample from a 55-year-old patient with untreated Parkinson disease is a good example of the typical micrographic handwriting that is often characteristic of this condition. The handwriting samples shown in (B) and (C) are from a patient with essential tremor.B: Prior to treatment, the sample shows the typical large, sloppy script. C: This sample, taken from the same patient with essential tremor while being treated with propranolol (160 mg/day), shows obvious improvement. Changes in written script can provide excellent clues to the type of movement disorder that is present (see Chapter 13). |
The elucidation of this history may make the diagnosis of parkinsonism evident. Not all patients will present with all of these symptoms, and a patient will occasionally present with only a single symptom and will yet have parkinsonism. Inquiring whether the onset of symptoms was abrupt or insidious; whether there has been a gradual progression of symptoms; whether there is a family history of neurologic syndromes; and whether there is concurrent drug use, past history of encephalitis,
or exposure to various toxins, including the use of street drugs, may help determine the etiology of the syndrome.
or exposure to various toxins, including the use of street drugs, may help determine the etiology of the syndrome.
Resting tremor is the most frequent presenting sign in these patients. The appearance of this tremor often precipitates the patient’s visit to the doctor. Parkinsonian tremor is highly characteristic and consists of a low-to-medium amplitude, with four to five cycles per second alternating movement. Tremor is defined as the involuntary rhythmic oscillatory sinusoidal movement that results from the alternating or synchronous contractions of reciprocally innervated antagonistic muscles. Resting tremor has been described as “pill rolling” because of the movement of the fingers and thumb. Its appearance resembles the activity of an “old time” pharmacist preparing a pill. The tremor, however, may begin in the hands, legs, or face and most often appears unilaterally in a single limb. It will often progress to involve the second limb of the same side before becoming bilateral. Tremor is the initial presenting symptom in 75% of patients. With the exception of impaired postural reflexes, the major signs of parkinsonism usually appear unilaterally. Careful observation of the tremor will reveal that it is a resting tremor that is ameliorated with purposeful movement. A simple way of assessing whether a tremor is primarily resting, postural, or kinetic is to have the patient perform the finger-to-finger and finger-to-nose maneuver and to observe the affected limb at rest, with outstretched posture and with movement. The patient with a resting tremor will have a marked amelioration of tremor when the arm springs into action. The patient with kinetic tremor will have no tremor at rest but will typically develop increased tremor as the hand approaches the target. When the resting limb is raised to an outstretched position, the tremor of Parkinson disease will diminish, although with maintained posture, the tremor may reappear until movement is initiated again.
 FIGURE 12.3 Moderate simian posture in a patient with Parkinson disease. Note the flexion of the upper extremities, upper trunk, and head. Facial masking is also apparent. |
When the limb is totally supported and at rest, the patient with resting tremor will be seen to have the tremor, whereas those patients with kinetic tremor will not. Although resting tremor is a common early sign, tremor is rarely a source of disability.
Cogwheel rigidity is a sign that can be present either unilaterally or bilaterally, depending on the stage of illness. The patient does not complain of “cogwheeling.” This sign is elicited by passive movement of the limb or neck through a full range of motion. When present, this sign is best elicited by slow flexion and extension of the wrist or neck. In addition to increased tone, a characteristic ratchetlike sensation is appreciated by the examiner with passive movement. There are some patients in whom the initial symptomatology is cervical or low back discomfort, and the question of whether increased muscle tone is responsible for this symptom has been raised.
Bradykinesia is responsible for much of the disability associated with parkinsonism. Slowness of voluntary movement contributes to increasing difficulty with the activities of daily living such as getting in and out of a car, rising from chairs, cutting meat, preparing food, dressing, and walking. Some of these difficulties can be easily observed during an examination by watching the patient rise from a chair, walk to the examining room, and undress. Gait impairment accounts for the greatest proportion of the emerging disability, as severe gait impairment results in a loss of independence in many of these activities of daily living.
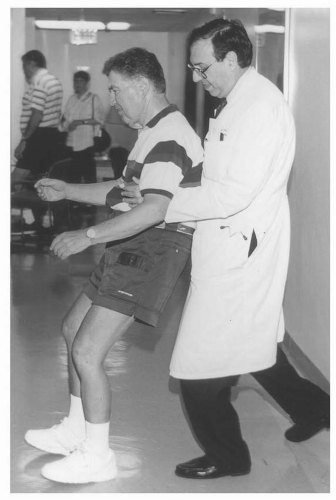
Postural reflexes refer to the ability of the patient to right himself and to keep from losing balance when sustaining postural perturbations (e.g., being jostled in a crowd). In addition, these reflexes are also important when turning around and changing direction while walking without losing balance. These reflexes can be evaluated simply and effectively by observing the patient walk 10 to 15 steps and turn around. A patient with normal postural reflexes should be able to pivot and turn without taking extra steps. In parkinsonism, one will often observe that the patient takes three to five steps to change direction. Another test of postural reflexes during the office visit is a firm backward pull on the shoulders or chest with the admonition to the patient that he should attempt to stop his backward motion in one to two steps. The examiner must be positioned behind the patient during this maneuver and prepared to stop the retropulsive movement or prevent a fall if necessary (Fig. 12.4). Impaired postural reflexes with frequent falls are a source of severe disability and injury in advanced parkinsonism (e.g., subdural hematoma, fractures of the hip or wrist). It should be noted that when postural reflex impairment is a prominent
early sign of parkinsonism, the diagnosis more often than not is not true Parkinson disease but instead one of the other neurodegenerative disorders that produces parkinsonism, such as progressive supranuclear palsy (PSP) or multiple system atrophy (MSA).
early sign of parkinsonism, the diagnosis more often than not is not true Parkinson disease but instead one of the other neurodegenerative disorders that produces parkinsonism, such as progressive supranuclear palsy (PSP) or multiple system atrophy (MSA).
There is no single pathognomonic sign of Parkinson disease; instead, the informed and experienced clinician uses a constellation of symptoms and signs to make the diagnosis.
There is also no known biologic marker of Parkinson disease, and there is no definitive laboratory or imaging study to confirm the diagnosis. Furthermore, identifying parkinsonism by history and clinical examination does not imply that the diagnosis is Parkinson disease, which is characterized by a specific neuropathology. Patients with a minimum of two of the four cardinal symptoms (tremor, rigidity, bradykinesia, and impaired postural reflexes) in the absence of this specific neuropathology are diagnosed as having an akinetic rigid syndrome or parkinsonism, the most common cause of which is Parkinson disease. Clinical features that help distinguish Parkinson disease from other forms of parkinsonism include unilateral presentation of symptoms and signs, slow progression, resting tremor, and responsiveness to dopaminergic agents.
MECHANISMS OF DISEASE
Parkinson disease is defined pathologically by the loss of dopaminergic neurons in the substantia nigra pars compacta and the presence of intracytoplasmic Lewy bodies, which stain for á-synuclein. The idiopathic degeneration of these neurons leads to loss of dopaminergic input to the corpus striatum. The progressive failure of the nigrostriatal pathway results in the symptoms of Parkinson disease. Neuropathologic examination of the brain of a patient with a history of Parkinson disease reveals loss of the pigmented neurons in the substantia nigra and loss of dopamine in the striatum where the nigrostriatal fibers project. Olfactory dysfunction, constipation, and rapid eye movement (REM) sleep disorder may represent early nonmotor manifestations of Parkinson disease, but this remains more speculation than fact.
The etiology of Parkinson disease remains elusive. Although numerous epidemiologic studies have investigated Parkinson disease, definitive environmental factors have not been identified. Nevertheless, there are many leads, and the presence of significant environmental toxin exposure in genetically predisposed individuals remains a
strong possibility. Inherited forms of Parkinson disease have been identified, the most common of which is leucine-rich repeat kinase (LRRK2) autosomal dominant Parkinson disease. LRRK2 mutations account for 10% to 40% of sporadic and inherited Parkinson disease in the Ashkenazi Jewish and North African Arab populations and lead to a typical, adult-onset Parkinson disease phenotype. Other autosomal dominant (á-synuclein) and autosomal recessive (parkin, PINK1, DJ-1) forms of Parkinson disease lead to young-onset Parkinson disease. However, surveys of large numbers of patients seen in movement disorder centers do not reveal a family history of Parkinson disease in the majority of patients with Parkinson disease. Nonetheless, identifying the á-synuclein and Parkin mutations has led to the discovery that the Lewy body is partly composed of α-synuclein and to the potential role of the proteosome in the pathogenesis of Parkinson disease. These recent genetic discoveries are very important and have opened the door to the concept that Parkinson disease may be a complex genetic disease.
strong possibility. Inherited forms of Parkinson disease have been identified, the most common of which is leucine-rich repeat kinase (LRRK2) autosomal dominant Parkinson disease. LRRK2 mutations account for 10% to 40% of sporadic and inherited Parkinson disease in the Ashkenazi Jewish and North African Arab populations and lead to a typical, adult-onset Parkinson disease phenotype. Other autosomal dominant (á-synuclein) and autosomal recessive (parkin, PINK1, DJ-1) forms of Parkinson disease lead to young-onset Parkinson disease. However, surveys of large numbers of patients seen in movement disorder centers do not reveal a family history of Parkinson disease in the majority of patients with Parkinson disease. Nonetheless, identifying the á-synuclein and Parkin mutations has led to the discovery that the Lewy body is partly composed of α-synuclein and to the potential role of the proteosome in the pathogenesis of Parkinson disease. These recent genetic discoveries are very important and have opened the door to the concept that Parkinson disease may be a complex genetic disease.
The demonstration in 1967 that orally administered levodopa could produce dramatic improvement in the symptoms of Parkinson disease was a remarkable therapeutic advance. For levodopa to have a therapeutic effect, it must cross the blood-brain barrier and be decarboxylated to dopamine. The enzyme that decarboxylates dopa to dopamine is ubiquitous and also decarboxylates several other aromatic amino acids. This enzyme, termed “aromatic amino acid decarboxylase” or “dopa decarboxylase,” is found in several extracerebral locations, including the gastrointestinal tract, liver, and kidney. When orally administered, levodopa is absorbed; acted on by the extracerebral decarboxylase; and converted to dopamine, which cannot cross the blood-brain barrier. If levodopa is administered alone, enormous quantities are required to overcome the peripheral decarboxylase systems and achieve a therapeutic benefit. The use of a peripheral decarboxylase inhibitor (carbidopa) with levodopa results in a marked reduction in the dose of levodopa necessary to achieve a central effect.
Another enzyme that plays a role in determining how much levodopa circulating in the blood reaches the brain is catechol-O-methyltransferase (COMT). COMT methylates levodopa and also reduces the concentration of levodopa that is available for transport across the blood-brain barrier. Peripheral COMT inhibition is another therapeutic maneuver to enhance levodopa bioavailability in the brain to increase central dopamine activity.
An additional enzyme that affects central levodopa availability is monoamine oxidase B (MAO-B). MAO-B acts by deaminating dopamine, leading to reduced availability in the synaptic vesicles. Central MAO-B inhibition is yet another therapeutic target to ameliorate symptoms of Parkinson disease by increasing levodopa bioavailability.
Because increasing the concentration of dopamine in the striatum results in dramatic clinical improvement in patients with Parkinson disease, the neural substrate that dopamine acts on (the striatal dopamine receptors) is obviously relatively intact. The dopamine receptor sites are divided into five different subtypes, but there are two main families: the D1 and D5 group and the D2, D3, and D4 group. Drugs acting as dopamine receptor agonists must have D2 activity to be effective in the treatment of Parkinson disease. The dopamine receptor agonists that are available to treat Parkinson disease include ergotderived (bromocriptine and pergolide) and non-ergot-derived (pramipexole, ropinirole, and rotigotine) compounds. Although all of the dopamine receptor agonists have D2 activity, they have somewhat different profiles of activation for the various dopamine receptors. Although all agonists have similar efficacy and adverse event profiles, there are variations among individual patients in regard to which agonist is the most efficacious. Although the number of antiparkinsonian medications grows steadily, levodopa remains the
gold standard of therapy, the most potent drug for the symptomatic treatment of Parkinson disease.
gold standard of therapy, the most potent drug for the symptomatic treatment of Parkinson disease.
TREATMENT
All medications currently used to treat Parkinson disease only provide symptomatic relief and do not alter the underlying pathogenesis of the disorder (Table 12.1).
In other words, the natural progression of Parkinson disease continues despite current treatment. The treatment of each patient with Parkinson disease should be highly individualized to provide functional improvement. If a patient’s symptoms are very mild and causing no impairment in the activities of daily living, delay of treatment may be the appropriate choice. If a patient’s symptoms are mildly troublesome, with tremor as the predominant feature, lowdose anticholinergics (e.g., trihexyphenidyl, benztropine) may be all that is required. The anticholinergics, the oldest drugs available to treat Parkinson disease, remain useful because the striatum contains high levels of both dopamine and acetylcholine, and the dopamine deficiency state in the striatum of patients with Parkinson disease results in a relatively elevated cholinergic tone. Anticholinergics exert their beneficial effect by partially correcting this relative cholinergic excess. Anticholinergics must be used with caution in older patients because they can induce memory dysfunction and confusion. In older men, anticholinergics can also lead to urinary hesitancy and retention.
TABLE 12.1 Medications Used in the Treatment of Parkinson Disease | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Amantadine is also useful in the treatment of early Parkinson disease and can be helpful for early bradykinesia. Amantadine has anticholinergic activity, mild dopaminergic activity, and antiglutaminergic activity. Amantadine is also useful in treatment of drug-related dyskinesia in more advanced Parkinson disease. Other options for patients with early troublesome symptoms include monotherapy with the dopamine receptor agonists (pramipexole, ropinirole, or rotigotine), or low-dose carbidopa-levodopa with or without a COMT inhibitor (tolcapone or entacapone). The progression of the disease process will eventually result in the need for more powerful dopaminergic stimulation.
The use of levodopa as a precursor loading strategy to increase central dopamine and to ameliorate Parkinson disease has been one of the major therapeutic advances in neurology. However, high-dose levodopa administration without the addition of a peripheral dopa decarboxylase inhibitor, such as carbidopa, produces anorexia, nausea, and vomiting. These symptoms occur because of the high levels of circulating peripheral dopamine that are present as a result of extensive extracerebral decarboxylation and result in the stimulation of the area postrema (emesis center) of the brain. The development of peripheral dopa decarboxylase inhibitors in large part ameliorated these problems and led to the development of combination therapy with carbidopa and levodopa. This drug is available in fixed ratios of 10/100, 25/100, and 25/250, with the numerator indicating the milligram dose of carbidopa and the denominator indicating the milligram dose of levodopa. The most important advantage of this drug is the ability to administer less
levodopa to obtain the same central effect with a marked reduction in nausea and vomiting. In fact, the ease of administration of carbidopa-levodopa therapy both for patient and the treating physician has resulted in it being used almost exclusively in the treatment of patients with Parkinson disease who require levodopa. Carbidopa-levodopa in a controlled-release (CR) formulation (CR 25/100, CR 50/200) provides a slower and longer-lasting effect of levodopa. CR preparations may be useful to treat motor fluctuations, nighttime bradykinesia resulting in sleep disruption, early morning painful dystonic cramps, and early morning severe bradykinesia. There is also a triple combination (levodopa/carbidopa/entacapone) tablet to treat Parkinson disease, which will allow some patients to take fewer pills daily. Levodopa remains the most potent drug for the treatment of bradykinesia and rigidity in Parkinson disease.
levodopa to obtain the same central effect with a marked reduction in nausea and vomiting. In fact, the ease of administration of carbidopa-levodopa therapy both for patient and the treating physician has resulted in it being used almost exclusively in the treatment of patients with Parkinson disease who require levodopa. Carbidopa-levodopa in a controlled-release (CR) formulation (CR 25/100, CR 50/200) provides a slower and longer-lasting effect of levodopa. CR preparations may be useful to treat motor fluctuations, nighttime bradykinesia resulting in sleep disruption, early morning painful dystonic cramps, and early morning severe bradykinesia. There is also a triple combination (levodopa/carbidopa/entacapone) tablet to treat Parkinson disease, which will allow some patients to take fewer pills daily. Levodopa remains the most potent drug for the treatment of bradykinesia and rigidity in Parkinson disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree