Figure 95.1. General approach for the management of organ donor.
CI = cardiac index; >CVP = central venous pressure; ELWI = extravascular Leng water index; SVR = sistemic vacular resistance
Complex monitoring with Swan-Ganz catheters (currently not used) or pulse-induced contour cardiac output (PiCCO) (widely used) affords advantages if they have been inserted beforehand or if you want to optimize the management of these donors in cases of severe or mixed hemodynamic instability (Figure 95.2).
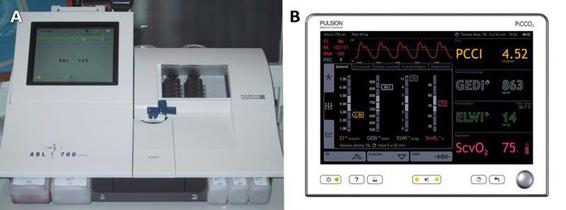
Figure 95.2. Apparatus necessary for the control of the donor. (A) Control electrolyte, blood gases and tissue ischemia by laboratory determinations at the bedside. (B) Control the hemodynamic state using the PiCCO technique.
Continuous temperature measurement with external systems is sufficient, although internal probes can be used to measure core temperature.
Another control is to closely monitor lung function: pulse oximetry ensures continuous oxygenation in these circumstances. PET CO2 is no longer necessary as brain protective ventilation has been stopped.
It is also important to monitor laboratory parameters such as glucose, lactate, and blood count values that not only detect blood loss but also hemodilution and coagulopathy. Also important is the determination of blood group, isogroups and Rh factor because blood may occasionally be needed. Determining electrolytes in blood and urine and blood gases is essential for monitoring the internal environment and preventing acid-base deficiencies or excesses that may impair graft viability. Specific parameters in relation to different organs are expressed in profiles: liver, kidney, with the calculation of creatinine clearance, the pancreatic profile and the cardiac profile.
When changes are happening quickly, serial determinations by decentralized basic acute care services are key to achieve donor stabilization before transfer to the operating room.
95.2.4 Management of Organ Systems
Hemodynamic Control
This is certainly the most important element to ensure good viability of organs for transplantation. Hemodynamic disorders subsequent to brain death are related to neurogenic shock that determines the loss of vascular resistance and causes relative hypovolemia, but also it can also be the result of polyuria due to diabetes insipidus.
Finally, the administration of hypertonic solutions (mannitol or hypertonic saline) for the treatment of intracranial hypertension can induce excessive diuresis. This situation triggers hypernatremic dehydration in most cases, unless the physician is aware of the changes occurring in the donor.
Not recommended is the treatment of the Cushing effect that precedes the final hypotensive phase since it is difficult to handle and because there is little or no pharmacological response. The ultrashort agent beta-blocker esmolol decreases the hypertensive and arrhythmogenic response. It is preferred when associated with tachyarrhythmias. Labetalol (alpha and beta-blocker) has a longer half-life and poor implementation. Another option s is hypotensive agents in patients with brain pathology, for example, alpha-blockers with controlled duration (e.g., urapidil) since they don’t affect cardiac rhythm or mask the bradycardia secondary to uncal herniation (Table 95.1). Despite the usually very transient crisis, drug administration may compromise hemodynamic stability, so further hypotension is the rule.
For the hypotensive phase, fluid administration is the first choice, guided by patient monitoring. Provide all necessary actions to maintain central venous pressure between 10 and 12 cmH2O. In most cases vasoactive amines will be needed. The type of fluids to infuse should be related to the patient’s osmolar state.
In cases of hypernatremia, we must administer hypotonic solutions such as dextrose (dextrose 5%) or hyposaline (0.45%) to adjust serum sodium. In the remaining cases, we use isotonic saline (0.9%) or Ringer’s lactate, adding as a regular basis colloids in a proportion of 1:3.
It is not uncommon in blood pressure management to administer amines, including norepinephrine (preferable) or dopamine up to 10-12 μg/kg/min. We adhere to these dose schedules to avoid causing tissue damage especially in the heart. In chest trauma with proven cardiac contusion, inotropes such as dobutamine up to 15 μg/kg/min as the maximum dose may be necessary, while taking care not to cause excessive vasodilation, which reduces diuresis. It may be necessary at times to use a combination of vasoactive drugs to prevent the deleterious effects of hypotension especially in the elderly, where the kidney is perfused at high pressure, to ensure that urine flow is maintained >100 ml/h. Adrenaline 0.1 μg/kg/min may be a viable alternative to excessive norepinephrine requirements in addition to improving hepatic blood flow. The administration of corticosteroids, thyroid hormones or vasopressin, which now is increasingly in disuse, can achieve hemodynamic stabilization in some cases (Table 95.1).
Drug/substance | Half-life | Bolus | Perfusion | |
Hemodinamics | Esmolol | 8 minutes | 250 µg/kg in 1 min. | 25-50 µg/kg/min |
Labetalol | 4 hours | 50 mg in 2 min | 1-2 mg/min | |
Urapidil | 4.7 hours | 25-50 mg in 20 sec | 2 mg/min | |
Vasopresin | 19-20 minutes | 1U | 0.01-0.03 U/min | |
Antiarrhythmics | Dopamine | 2 minutes | – | 5-10 µg/kg/min |
Adrenaline | 2 minutes | – | 0.1-1 µg/kg/min | |
Isoproterenol | 5 minutes | – | 2-10 µg/kg/min | |
Hormones | Desmopressin | – | 0.03-0.15 µg/kg | |
Triiodothyronine (T3) | 4 µg | 3 µg/h | ||
Tyrosine (T4) | 20 µg | 10 µg/h | ||
Methylprednisolone | 15 mg/kg/day | |||
Table 95.1. Dosage and half-life of drugs and hormones in the management of donor organs.
Vasopressin at a vasopressor dose (1-2 IU/h) in conjunction with other amines increases resistance in a similar way as used in acute spinal cord injury.
Before transferring the patient to the operating room for organ removal, the surgical team should be informed of amine requirements.
Control of Arrhythmias
The brain-dead organ donor is very susceptible to develop brady- or tachyarrhythmias, atrial or ventricular in origin, as well as different degrees of heart conduction blocks.
Bradyarrhythmias are generally self-limiting, reversible, and do not require treatment; however, if you do need a pharmacological agent, the choice is dopamine at beta-dose; and if no response is obtained, epinephrine or isoproterenol may be tried. Rarely, the need for temporary pacemaker implant may be considered (Table 95.1).
For tachyarrhythmias and other conduction abnormalities, after ruling out etiological causes of cardiac or extracardiac origin, amiodarone should be considered the treatment of choice. Up to 10% of donors may have cardiac arrest in which cardiopulmonary resuscitation (CPR) can be performed according to international standards, except for the use of atropine due to central denervation of the heart muscle since brain death prevents the heart from responding to this drug. If CPR is unsuccessful and the donor remains in cardiac arrest, he or she can be transferred immediately to the operating room under breathing bag-assisted breathing and chest compressions for the removal of organs suitable for donation; in hospitals with implemented protocols heart-beating donation should be applied immediately.
Respiratory Control
In the brain-dead patient, hypoxemia of any origin should be avoided and the lungs must be protected by so-called “protective ventilation”, especially if the patient is younger than 50 years of age, with no history of smoking or bronchopulmonary diseases that exclude the likelihood of organ donation. The hyperoxia test to check gas exchange values will be made by ventilation with oxygen at 100% and PEEP of 5 cmH2O for 5 minutes reaching a PaO2 >300 mmHg. If this test confirms good gas exchange, mechanical ventilation will be <3 days, and we can confirm the possibility by measuring chest circumference and chest radiology without infiltrates: all such parameters can help to predict a successful lung transplant and exitus care in preserving the lungs.
Protective ventilation entails limiting tidal volumes, so that the machine is set to 6 ml/kg with FiO2 necessary to maintain pulse oximetry ≥95%, generally 50% or lower to decrease the possibility of oxygen toxicity. This objective will be sufficient to maintain optimal function. The PEEP is set at 5 cmH2O and peak inspiratory pressures are kept ≤30 cmH2O.
Since metabolic needs decrease in the donor brain-dead patient, CO2 should be not a problem. It is not uncommon that donors are hyperventilated, so serial blood samples should be taken every 4 hours during the time of preservation.
In atelectasis, the recommendations of the Acute Respiratory Distress Syndrome (ARDS) Network Working Group should be followed, handling PEEP according to the inspiratory fraction of oxygen (Table 95.2).
PEEP (cmH2O) | FiO2 (%) |
5-10 | 60 |
10-15 | 80 |
15-20 | 100 |
Table 95.2. PEEP and FiO2 values.
These values should not be exceeded; if peak pressures are >45 cmH2O during recruitment, controlled pressure ventilation will be necessary.
The excessive positive fluid balance will be controlled with diuretics; this is not usually frequent unless treated with donors with suboptimal pathology control or without organic reserve. In young people there is often a loss of water due to endocrine disruption. Such patients should be managed with PiCCO for volume management.
Decreased tidal volume can also produce atelectasis. This occurs due to a loss of volume of the alveoli and subsequent collapse. Re-expansion with the standard apnea test can provoke atelectrauma. This forces us to introduce PEEP regularly. If the lungs prove sensitive, we can apply the variant T-tube and PEEP valve or, if the patient is on an adapted ventilator, spontaneous breathing modes such as continuous positive airway pressure (CPAP) to the lung can be induced. Aspirations of secretions should be done carefully with an aseptic technique without depressurizing the system in closed suction systems.
In patients who had suffered significant bronchoreactivity, high doses of steroids or beta adrenergic drugs can be given to solve the problems of mast cell degranulation and precipitation of the crisis. However, the use of steroids and adenine-vasopressin agents implies restricting the volume of fluid to infuse.
The administration of prophylactic antibiotics is mandatory, especially in donors eligible for lung transplant or who remain >48 hours in the ICU. Cultures at an early stage should be obtained, and broad-spectrum antibiotics administered such as amoxicillin-clavulanate, cephalosporins, quinolones, or those that have been formalized in the ICU in relation to the prevalent flora.
Endocrine Control
A wide range of changes in hormonal pathways occur after brain death. The brain is a great central controller, principally localized in the hypothalamus.
Diabetes Insipidus
Vascular ischemia secondary to circulatory arrest in the context of cerebral herniation and intracranial hypertension results in the destruction of the hypothalamus and the axis that regulates the secretion of ADH released from the neurohypophysis. Biphasic and triphasic patterns that may occur in surgical patients or in patients with Sheehan syndromes are not often encountered, where within about 6 hours all hormone traces disappear, although the presence of the symptoms can be manifest with ADH levels of 10-90% of the normal range. The result is hypotonic polyuria with a urine output of 4 ml/kg/h, urine density <1005, hyponatremia, plasma hyperosmolality, usually >300 mOsm/kg and urinary osmolality <300 mOsm/kg. This water loss not only carries away sodium but also provokes the loss of potassium, magnesium, phosphorus and calcium. All these alterations must be corrected.
If urine output is >5-7 ml/kg/h and electrolyte control is difficult to achieve, treatment with vasopressin analogues is necessary. This can be given as vasopressin or desmopressin, the latter is now the treatment of choice because of its excellent action and almost no pressor action. Desmopressin has an antidiuretic action 2000 times more potent than vasopressin and its action is 3 to 5 times longer. The administration can be intranasal, intramuscular or intravenous. Vasopressin is only indicated in the presence of severe hypotension resistant to treatment with fluids or amines. The desmopressin dose varies between 0.03 to 0.15 μg/kg titrated to maintain urine output between 2 and 3 ml/kg. Usually, the pharmaceutical presentation is 1-ml vials containing 4 μg of desmopressin. Nasal instillation drops are used in the treatment of chronic deficits.
The undesirable effect is mainly water intoxication. Hours after desmopressin administration there is a dramatic reduction in diuresis which can result in severe dilutional hyponatremia with hemodynamic overload, leading to heart failure. If vasopressin is used, vasoconstriction is a potentially dangerous side effect because it can trigger severe bronchospasm and ischemia of various territories including the myocardium, splanchnic and renal peripheral areas among others.
Glucose Metabolism
The adrenergic and metabolic crisis that ensues after brain death leads to moderate hyperglycemia which can sometimes become severe due to other reasons such as: administration of catecholamines to maintain blood pressure, administration of glucose to maintain normal osmolarity or decrease serum sodium, hypothermia; the use of steroids is another factor involved. Some studies have failed to demonstrate a lack of insulin production in potential donors: the levels may be low, normal or increased.
The major pathophysiological consequences of hyperglycemia are metabolic acidosis, ketosis, hyperosmolarity (of the extracellular environment that leads to intracellular dehydration) and electrolyte changes. Furthermore, hyperglycemia causes osmotic diuresis with polyuria and urinary loss of water and electrolytes contributing to hypovolemia and therefore hemodynamic instability. We must initiate rapid intravenous insulin treatment by continuous infusion to maintain blood glucose between 150 and 200 mg/dl. Some studies have reported a negative action on pancreatic beta cells, so they should be closely monitored in pancreas donors.
The treatment with short-action insulin is indicated and, if necessary, will be maintained with continuous infusion for better control. The control in cases of instability must be done frequently, and if pumps are used to maintain dosing schedules the recommended target is between 80 and 150 mg/dl. Infusion will be administered according to the protocols in place in the ICU; glucose levels >200 mg/dl should be maintained with 2 to 7 units of insulin per hour.
Alterations of the Hypothalamic-Pituitary-Adrenal and Thyroid Systems
It has been observed in studies by Novitzky, Schrader and Jeevanandam that levels of triiodothyronine (T3), tyrosine (T4) and cortisol are reduced after brain death. Although the levels of thyroid stimulating hormone (TSH), prolactin, growth hormone and luteotrophic (LSH) do not vary over baseline, the decrease in peripheral hormonal levels at 6 hours after brain death triggers a chain of events: high-energy phosphate depletion, increased lactate and free fatty acids that signal the change from aerobic to anaerobic metabolism, and mitochondrial failure. However, some studies showed that after the administration of TRH, TSH increases slightly, suggesting a preserved partial flow after the cessation of cerebral blood flow. All these phenomena result in organ deterioration (e.g., heart and kidneys).
Some authors have reported that the decrease in thyroid hormones detected after brain death is accompanied by a fall in cortisol and insulin levels which can occur in other pathological conditions such as head trauma, severe sepsis, burns, and the so-called sick euthyroid syndrome. This syndrome is characterized by lower T3, accompanied by high levels of rT3; tyrosine (T4) may be normal, high or low, showing a defect in the conversion of T4 to T3 in the periphery, while maintaining normal levels of TSH, so the hormonal profile is configured as a criterion to establish euthyroidism.
It is important to note that alterations in heart rate, bradyarrhythmias resistant to medication or hypotension refractory to inotropes can be treated with thyroid hormone or corticosteroid replacement. Replacement therapy can be initiated as follows: intravenous bolus of 4 μg of T3, continued with an infusion of 3 μg/h. The alternative is T4 which requires a bolus 5 times larger and then continuous infusion of 10 μg/h in conjunction with steroids: bolus of 15 mg/kg methylprednisolone which can be repeated daily while maintaining hydrocortisone, which has a very short duration: 2 hours of efficient action after a dose of 100 mg of the drug, so the administration must be repeated (Table 95.1).
Electrolyte Control
One of the changes immediately after brain death is secondary endocrine failure. Alterations derived from the loss of regulation of the hypothalamic-pituitary axis must be monitored closely. Such alterations usually occur almost immediately. The loss of free water in the urine carries an increased loss of potassium which, unless properly replaced, can generate severe and life-threatening disturbances with critical implications principally for the heart and the potential loss of the donor. To avoid this complication, serial determinations are performed. If the potassium concentrations fall below critical values, replace immediately with the necessary amount of milliequivalents.
It is not unusual at times and in relation to hormonal correction to find hyperkalemia which also may occur secondary to cytolysis due to long-term maintenance or renal failure and metabolic acidosis. In such cases, the levels may rise quickly and unexpectedly. Hemolytic states, occasionally subsequent to blood transfusion, can increase potassium levels and require urgent treatment. Other causes of hypokalemia include: administration of glucose and insulin to facilitate potassium entry into the cell, 1 mol sodium bicarbonate infusion, beta-stimulants, calcium gluconate and loop diuretics such as furosemide.
Serum sodium disorders are very common: hypernatremia is especially frequent usually multifactorial in origin.
It’s very common to find hypernatremia prior to brain-death diagnosis, principally due to the treatment of intracranial hypertension with sodiumHypertonic solutions. In cases it is a result of diabetes insipidus with concomitant contraction of blood volume. This alteration is especially harmful for liver function and may compromise subsequent graft take. The treatment is with hypotonic infusions such as dextrose or hyposaline solutions.
Hyponatremia is less frequent but can ensue from inadequate intake, inappropriate correction of hormonal disorders, gastrointestinal losses or kidney failure. Treatment is based on fluid restriction and isotonic saline administration with serial laboratory monitoring.
Less frequent are alterations in magnesium, which often occur with refractory hypokalemia, both preventable with the administration of magnesium in conjunction with phosphate to preserve cell membrane integrity.
Temperature Control
The progressive destruction of the hypothalamus leads to a loss of body temperature in relationship to the external environment, with the patient becoming a poikilotherm donor. We must therefore prevent the dispersion of heat by mean of heating systems and fluid replacement. Hypothermia can generate hemodynamic instability, cardiac arrhythmias, renal and coagulation alterations and deviation of the dissociation curve of hemoglobin to the left.
Infused solutions, as well as ventilation gases, should be heated with humidifiers and inspired gas heaters, thermal electric blankets, all directed to maintain a core temperature of 35°C.
Hyperpyrexia should be avoided; in patients who had infections we must ensure negative cultures, negative screening for emboligenic foci from the heart and other locations, and adequate antibiotic treatment before authorizing organ donation.
Hematological Control
The origin of hematological disturbances is multifactorial: it may be due to severe blood loss in multiple trauma, previous transfusions with citrated blood, hypothermia and the continued release of large amounts of tissue fibrinolytic factors, as well as severe skull base trauma, multiple fractures internal organ (liver) injuries, major pelvic fractures or severe leg damage. Furthermore, ischemic-necrotic tissues can release great amounts of thromboplastin. There may also be non-specific abnormalities due to disseminated intravascular coagulopathy. Treatment is based on the administration of plasma and platelets and packed red cells if the hematocrit is <30% for kidney donors or 35% for multiorgan donors.
Renal Function
It is necessary to maintain an hourly urine output of 1 ml/kg in adult patients and 2 ml/kg in children. Polyuria, (urine output >3-4 ml/kg) can perpetuate a situation of hypovolemia due to diabetes insipidus or hyperglycemia.
If the donor is oliguric, with urine output <0.5 ml/kg/h despite maintaining adequate blood pressure and appropriate central venous pressure, administration of diuretics will be necessary. The most frequently used include furosemide at doses of 20-60 mg and intravenous mannitol at doses of 0.25-0.50 g/kg.
Specific Corneal Care
Exclusion criteria for cornea donation are the same as general criteria. We should specifically take care of the donor’s eyes, trying to keep them moist and properly occluded. Periodically, lubricants, artificial tear solutions, antibiotic drops of tobramycin or gentamicin should be administrated. Occasionally, corneal epithelial alterations should be minimized with the administration of local cold.
95.2.5 Tests and Special Care for Organ Donation. Diagnostic Determinations in Intensive Care
General diagnostic tests are performed to monitor potential donors; however, to evaluate different viscera we must follow a series of requirements as described below. A general test is the determination of blood count and blood group with Rh factor and investigation of isogroups, which have been obtained by previous requirements. In addition, immunological compatibility between donor and recipient is necessary for heart, liver and lung transplants, as is determining serology. Determination of the type (typing) by extracting lymph nodes is not usually done in the ICU, but is now performed in the operating room.
Monitoring and Special Tests for Lung Donation
Previous care is essential to avoiding volutrauma and barotrauma in mechanically ventilated patients. Indispensable is serial monitoring of blood gases. Assessment will include previous infections, history of smoking, drugs, chemotherapy or radiotherapy, and evaluation of previous occupational risks.
Determination of specific laboratory profiles and arterial blood gases, pH, partial pressure of O2, CO2, and serum bicarbonate must be monitored every 4 hours. Before a patient can be considered as a potential lung donor, it is imperative to measure PaO2 >300 mmHg after the administration of 100% oxygen for 15 minutes with a PEEP of 5 cmH2O.
Chest radiography is important for lung assessment. Check for the presence of pneumonia, neurogenic or cardiogenic pulmonary edema, pneumothorax or hemothorax, which are highly prevalent in patients with severe head injuries under mechanical ventilation. Anthropometric assessment will include measurement of body weight, height, waist circumference and chest circumference.
Lung transplantation also requires measurement of the diaphragmatic dome-lung apex axis (B and C) and the largest thoracic diameter measured between both costophrenic sinuses (A) (Figure 95.3).
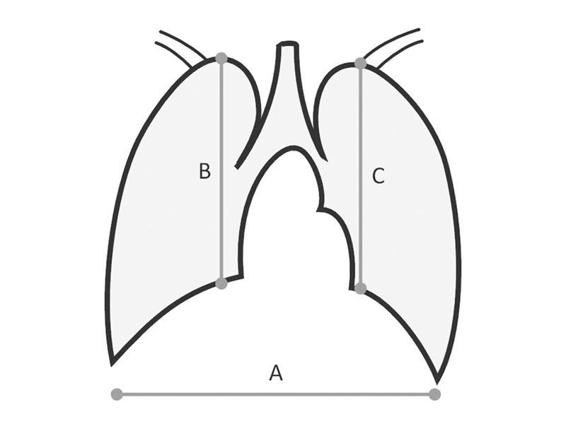
Figure 95.3. Measures to determine the suitability between donor and recipient: dome-lung apex axis (B and C) and the largest thoracic diameter measured between both costophrenic sinuses (A).
Monitoring and Special Tests for Heart Donation
It is essential to anticipate an adrenergic storm, which is not dangerous for the heart and will not provoke heart failure. Just as in the lung donor, we must search for salient events in the medical history and risk factors. An ECG is essential.
Determination of specific cardiac laboratory profiles: levels of CPK, CK-MB, troponin to assess possible heart damage, especially in cases of chest trauma, cardiac arrest and cardiac massage.
ECG is mandatory and as is coronary angiography in patients over 45 years of age, so we can assist the donor as quickly as possible.
Monitoring and Special Tests for Liver Donation
Complete and detailed medical history and determination of risk factors for liver disease.
Determination of the hepatic profile: levels of GOT, GPT, GGT, total and direct bilirubin, glucose, electrolytes and clotting times, prothrombin time, partial thromboplastin time and INR. Alterations in these enzymes may indicate chronic liver disease, hepatic necrosis secondary to tissue hypoperfusion due to complications arising from maintenance of hemodynamic status. Also important is the search for tumour markers such as alpha-fetoprotein associated with hepatocellular carcinomas and germ cell tumours.
Abdominal ultrasound.
Monitoring and Special Tests for Kidney Donation
Detailed clinical history and risk behaviours.
Renal profile by laboratory determinations should include: complete urinalysis, proteinuria, blood urea and creatinine.
Also indispensable is determination of chorionic gonadotropin (HCG) to search for choriocarcinoma, germ cell tumours (ovary and testicles), and tumours with ectopic secretion of HGH such as urinary bladder. Prostate specific antigen (PSA) as a marker of prostate cancer, has significance when the values are >10 mg/l.
Ultrasound assessment of the kidneys and excretory pathways.
Monitoring and Special Tests for Pancreatic Activity
Clinical history and risk behaviours are a common step to other organs, focusing on smoking habits, history of gallstones, and alcohol consumption.
The specific pancreatic profile for the laboratory includes: blood glucose and serum amylase and lipase, especially for assessing the viability of the pancreas for transplantation. Lipase levels are not usually affected by brain death, but blood glucose and amylase >10 mg/l are.
Ultrasonography of the pancreatic area; computed tomography (CT) is mandatory if there are any abnormalities in the parenchyma or their excretory pathways.
Key Concepts
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






