Radiopharmaceutical
Administered activity
Number of cycles
Time interval between cycles (weeks)
90Y-DOTATATE/90Y-DOTATOC
3.7 GBq
(100 mCi)/m2
2
10–12
90Y-DOTATATE/90Y-DOTATOC (another protocol)
2.78–4.44 GBq
(75–120 mCi)
2–4
10–12
177Lu-DOTATATE/177Lu DOTATOC
5.55–7.4 GBq
(150–200 mCi)
3–5
10–12
177Lu + 90Y
Combination-Cocktail protocol
5.55–7.4 GBq
(150–200 mCi)
(for 177Lu)
2.5–5 GBq
(68–135 mCi)
(for 90Y)
2–6
10–16
Treatment doses should be reduced and individualized for compromised patients according to clinical and biochemical parameters.
27.9.1 PRRT for Paediatric Patients
In children, 90Y-DOTATOC can be given in 1.5–1.85 GBq/m2 activities per cycle in up to 3 cycles. There is no widespread use of 177Lu-DOTATATE in paediatric patients, and activities should be adjusted per body surface area [7].
27.9.2 Retreatment with PRRT
Retreatment should be considered for patients with well-documented disease progression, who previously responded. The decision of retreatment should be taken within tumour board, and the same eligibility criteria apply like the first treatment. Retreatment can be done with the same or a different radiopeptide.
27.10 Imaging After PRRT
Since 177Lu emits gamma radiation in addition to therapeutic beta particles, scintigraphic imaging using gamma rays should always be done following each treatment cycle to confirm targeting of radiopharmaceutical and to assess functional response. Planar and SPECT and/or SPECT/CT tomographic images are acquired usually on days 1, 4 and 7 after PRRT with 177Lu-DOTATATE (Fig. 27.1a, b).
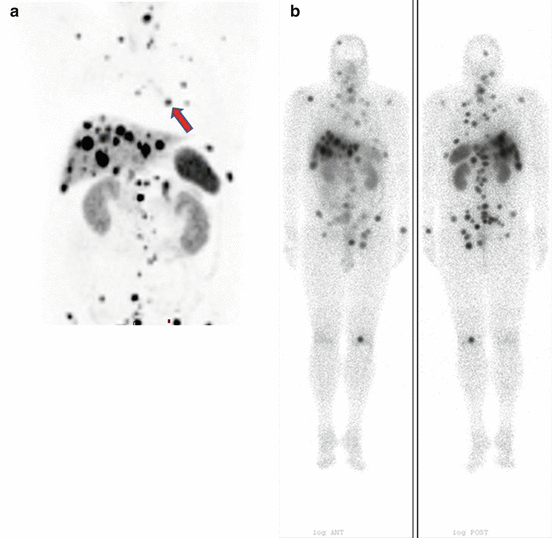

Fig. 27.1
(a) Whole-body maximum intensity projection (MIP) 68Ga-DOTATATE image of a 56-year-old male patient with the diagnosis of multiple hepatic neuroendocrine tumour metastases from an endobronchial carcinoid tumour primary (arrow) shows multiple foci of uptake in the liver, in the bones and in the thorax consistent with extensive metastatic disease and referred to PRRT. (b) Whole-body anterior (left) and posterior (right) 177Lu-DOTATATE posttherapy images show more number of lesions compared to diagnostic imaging
Since 90Y is a pure beta emitter, it does not permit direct scintigraphic imaging, but even if it is poor in quality, Bremsstrahlung (braking radiation) rays can be used to obtain a posttherapy imaging. It is usually done in 24 h following PRRT with 90Y-DOTATOC.
27.11 Outcomes of PRRT for Neuroendocrine Tumours
27.11.1 Response
Response evaluation for PRRT includes functional and anatomic images to assess functional and morphological response as well as biochemical and symptomatic response to assess patients’ quality of life. Posttherapeutic 177Lu-DOTATATE imaging can successfully be used to assess functional response between therapy cycles and at the end of the therapy. The time period for response evaluation depends on different situations according to clinical needs, but generally the first follow-up is recommended after 3 months and to be continued after 3–6 months.
PRRT for neuroendocrine tumours with 177 Lu- and 90Y-labelled somatostatin analogues has been in clinical use for more than a decade. Most of the studies in the literature conclude that PRRT can give high absorbed doses to neuroendocrine tumours with overexpression of sstr2 which results with partial and complete objective responses in up to 30 % of patients. Gastroenteropancreatic neuroendocrine tumours have showed the best objective response rates, with partial responses ranging from 9 to 29 % and complete remission rates from 2 to 9 %. Almost similar response rates have been reported for neuroendocrine tumours of the lungs and neuroectodermal tumours such as paragangliomas. Dedifferentiated thyroid carcinomas, medullary thyroid cancers and neuroendocrine tumours of the thymus have displayed less satisfactory response rates. Besides these, promising results have been reported for other sstr-positive tumour types such as astrocytomas, medulloblastomas and meningiomas [8–12].
27.11.2 Survival
According to some studies in which survival analyses have been reported, patients who have high somatostatin receptor expression at the beginning of PRRT show significantly higher objective responses in correlation with significantly longer survival when compared with similar patients with lower receptor expression [10, 13, 14].
Some studies have reported that biochemical response is also good predictor for the overall survival of patients with dedifferentiated thyroid cancer and medullary thyroid cancer following 90Y-DOTATOC therapy [9, 15]. Bushnell et al. concluded that significant symptomatic response after 90Y-DOTATOC therapy has had an impact on progression-free survival of patients [16].
27.11.3 Side Effects and Toxicity
The kidney is the dose-limiting organ for PRRT. Pretherapy renal protection protocols are mandatory, and they reduce renal absorbed doses significantly and avoid serious renal dysfunction due to PRRT [5, 17]. Renal toxicity is more frequently seen with 90Y-DOTATOC; especially the patients with the diagnosis of long-standing and poorly controlled hypertension have high risk for renal toxicity [16]. Despite kidney protection, renal dysfunction can occur following PRRT with approximate creatinine clearance loss of 3.8 % per year for 177 Lu-DOTATATE and 7.3 % per year for 90Y-DOTATOC [18].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






