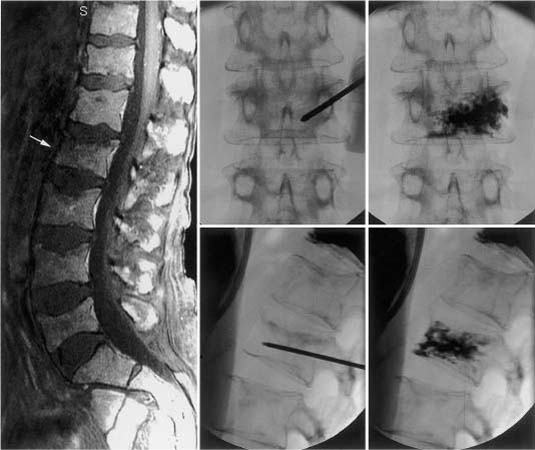
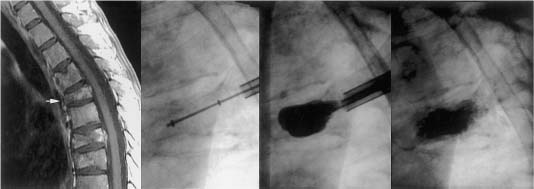
43 Developed in France in the late 1980s, minimally invasive vertebroplasty involves the percutaneous injection of polymethylmethacrylate (PMMA) into a fractured vertebral body.1,2 Although this does not reexpand a collapsed vertebra, reinforcing and stabilizing the fracture seem to alleviate pain (Fig. 43-1). Procedural complications are relatively rare; however, most are related to leakage of PMMA through cortical defects, with epidural compression of the neural elements.3 Percutaneous balloon kyphoplasty, a recent modification of vertebroplasty, involves inflation of a balloon into a collapsed vertebral body to restore height and reduce kyphotic deformity, prior to stabilization with PMMA.4–8 The risk of cement extravasation is theoretically reduced because inflation of the balloon creates a void within the vertebral body into which cement can be injected under relatively low pressure (Fig. 43-2). Vertebroplasty was first used to treat aggressive vertebral hemangiomas,1 and was later applied to other lesions that weaken the vertebral body, including myeloma bone disease and osteolytic metastases.9–11 In North American centers, vertebroplasty and kyphoplasty are most frequently used to treat painful osteoporotic compression fractures.12–15 However, the North American oncologic community is just beginning to embrace these techniques.7,8,16 It is hoped that this chapter will help foster a greater role for these procedures in the treatment of patients with nonosteoporotic benign and malignant vertebral destructive lesions, as this is the patient population that perhaps benefits most from the rapid and durable reduction in spinal pain that vertebroplasty and kyphoplasty offer.17 Although a mechanical mechanism seems most likely, the origin of pain relief provided by vertebroplasty or kyphoplasty is poorly understood. Other hypotheses cite the thermal18 and chemical19 effects of PMMA as perhaps causing tumor necrosis or destruction of the nerve endings in adjacent sensitive tissue. However, there is a growing consensus that stabilization of microfractures and a decrease in the mechanical stresses placed on the affected vertebrae play a significant role.10,16 In an ex vivo biomechanical study of osteoporotic spines subjected to axial compression tests, vertebrae injected with PMMA demonstrated, compared with noninjected controls, increased stiffness of 174% and increased maximal force required before failure of 195%.20 Some authors have argued that if the vertebral strengthening effect is the mechanism of pain relief, one would expect to find the degree of improvement to be proportional to the total volume of injected acrylic and the extent of vertebral filling.16 However, neither of these variables has been shown to correlate with pain relief.10 On the other hand, in another biomechanical study of osteoporotic spines where the dose—response effect of PMMA injection was analyzed, strength was restored to regions where as little as 2 mL of cement was injected.21 Critical issues are the durability and longevity of PMMA, although it has long been used by neurosurgeons for reconstruction of the vertebral body following corpectomy.22,23 Certainly, the monomer-to-powder ratio affects its compressive properties.24 When used for vertebroplasty or kyphoplasty, barium sulfate powder (for radio-opacification) and other “impurities” such as antibiotic powder are typically added to the mixture. However, even when altered by the addition of these materials,25 or by changing the monomer-to-powder ratio,24 the compressive strength of PMMA easily surpasses that of a vertebral body. The increased strength and stiffness provided by PMMA injection have in fact, led to some concern regarding changes in adjacent segment stress.26 FIGURE 43-1 L2 vertebroplasty in a patient with multiple myeloma. Preoperative sagittal magnetic resonance imaging (MRI) (left) shows osteolytic changes at L2 (arrow). Anteroposterior (top) and lateral (bottom) fluoroscopic images obtained during the procedure show the needle trajectory (middle) and final polymethylmethacrylate (PMMA) casting (right). (From Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003;98(1 suppl):21–30, with permission.) Vertebral hemangiomas are common benign spinal tumors that are usually asymptomatic. They are rarely painful. Close correlation between clinical and radiologic findings is required before spinal pain should ever be attributed to such lesions. In patients with symptomatic hemangiomas, pain reduction occurs in ~80% of those treated with vertebroplasty.1,11,27,28 Painful aggressive and destructive vertebral hemangiomas are extremely rare, but may cause neural compression secondary to compression fracture, hematoma, epidural extension of tumor, or bony expansion.29 The surgical alternative (i.e., vertebrectomy and reconstruction/stabilization) may be associated with horrendous blood loss despite preoperative transarterial embolization. Vertebroplasty for bone strengthening and direct embolization of the affected vertebral body, followed by decompressive laminectomy and resection of any epidural extension of the hemangioma, have been recommended.27,28 This combination of approaches may also be preceded by transarterial embolization of the feeding arteries. FIGURE 43-2 T6 kyphoplasty in a patient with multiple myeloma. Preoperative sagittal MRI shows T6 osteolytic compression fracture (white arrow). Sequential lateral fluoroscopic images show the trajectory of the inflatable bone tamps (IBTs), inflation of the balloons, and filling of the bony voids with PMMA. Two markers identify the balloon position for accurate placement (arrows). (From Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003;98(1 suppl):21–30, with permission.) The indications for vertebroplasty among other benign spinal tumors are rare. There is one report of transoral vertebroplasty for a fractured C2 aneurysmal bone cyst.30 Vertebroplasty of a previously radiated but progressively painful vertebral eosinophilic granuloma with instability of the spine has also been reported.31 The current nonsurgical treatment options for malignant spinal disease include analgesics, radiation therapy, hormones, cytotoxic drugs, embolization, and bisphosphonates.32 However, none of these treatments addresses axial (or mechanical) back pain due to vertebral body collapse or deformity. Axial pain is a significant cause of morbidity in patients with metastatic spinal tumors and myeloma bone disease. Spinal stabilization is very effective in relieving this type of pain.22 Because malignant pathology most typically involves the vertebral body, vertebrectomy and reconstruction of the anterior weight-bearing capacity of the spine are required. These surgical procedures require significant postoperative recovery time and are often associated with morbidity and mortality in patients with limited life expectancy. Vertebroplasty and kyphoplasty represent important additions to the therapeutic arsenal because these techniques can be used to address axial pain in patients who would not be considered surgical candidates because of limited functional capacity, short life expectancy, or multilevel spinal disease.8 Either vertebroplasty or kyphoplasty are most clearly indicated in the patient with well-localized disabling axial-type pain secondary to neoplastic thoracic or lumbar vertebral body fracture or collapse, without evidence of epidural disease.8,17 Because of the range of therapeutic options for patients with malignant spinal neoplasms, the decision to treat is made by a multidisciplinary team including a neurosurgical or orthopedic spine surgeon and a radiation or medical oncologist. In our experience, the early involvement of pain management and rehabilitation medicine specialists in the care of these patients is extremely helpful. The ultimate treatment plan for the individual patient depends on a variety of factors including the clinical presentation, duration of symptoms, tumor type, anticipated radiosensitivity, tumor location, extent of extraspinal disease, integrity of the spinal column, medical fitness, and life expectancy. Patients with significant epidural compression of the neural elements by tumor, bone, or disc fragments are not considered candidates for vertebroplasty or kyphoplasty. Other contraindications include failure to localize symptomatic level(s); pain that is predominantly local or radicular in nature, which may be addressed with surgical decompression or radiation therapy; and medical contraindications, such as uncorrected coagulopathy, local infection at the planned injection site, or intolerance to prone positioning. A lack of surgical backup is an absolute contraindication. Kyphoplasty, in our experience, should always be performed with the patient under general anesthesia; therefore, vertebroplasty is performed if the patient will not tolerate either the general anesthesia or the relatively longer procedural time required for kyphoplasty. Disruption of the posterior vertebral body cortex is considered a relative contraindication for vertebroplasty because of the increased risk for cement leakage. The risk theoretically may be reduced with the more controlled delivery of bone cement provided by kyphoplasty5 Extensive vertebral collapse (i.e., vertebra reduced to less than one third of its original height) makes both vertebroplasty and kyphoplasty more technically challenging to perform and therefore represents another relative contraindication. In such cases (especially for patients with vertebra plana deformity), kyphoplasty is preferred to restore height; however, vertebroplasty is performed when collapse is too severe to permit insertion of the balloon device.8 A treatment algorithm for vertebroplasty and kyphoplasty in cancer patients with painful thoracic or lumbar vertebral body fractures is presented in Fig. 43-3. FIGURE 43-3 Treatment algorithm for painful thoracic or lumbar vertebral body (VB) fractures in cancer patients. See text for details. RT, radiation therapy. (From Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg 2003;98(1 suppl):21–30, with permission.) Currently, the biomechanical effects of spinal tumors and the mechanisms of neoplastic vertebral collapse remain ill-defined. There are no clear standards for predicting the risk of pathologic fracture, even when lesions have been identified and characterized with bone scans, computed tomography (CT) scans, or magnetic resonance imaging (MRI). For metastatic tumors, vertebral body collapse may theoretically be prevented by radiation therapy if the lesion is radiosensitive and its growth (and therefore the lytic destruction of the vertebra) can be inhibited. Once the tumor reaches a critical size, which can be defined as “impending collapse,” only stabilization can prevent fracture. Several authors have advocated the use of vertebroplasty for prophylactic stabilization in asymptomatic patients with radiologic evidence of impending collapse.9,10,16 Certainly, some reliable method to identify impending collapse would be extremely beneficial. The few experimental studies addressing this issue have had limited success in developing an adequate model of osteolytic disease as well as the mechanisms to generate collapse.33–35 Taneichi et al36 analyzed radiologic and clinical data from 53 patients with 100 thoracic or lumbar metastases, using a multivariate logistic regression model in an attempt to identify the probability of collapse under various states of metastatic vertebral involvement. They found distinct differences in the timing and occurrence of vertebral collapse in the thoracic spine compared with thoracolumbar or lumbar regions. In the thoracic spine, destruction of the costovertebral joint was a more important risk factor for collapse than the size of the metastatic lesion within the vertebral body. This phenomenon was attributed to the loss of stiffness and strength normally provided by the rib cage. In the thoracolumbar and lumbar spine, the most important factor for collapse was the size of the vertebral body defect. In addition, metastatic involvement of the pedicle had a much greater influence on vertebral collapse in thoracolumbar and lumbar spine than in the thoracic spine. A major limitation of this study was that only tumor size and the location of defects within the vertebrae were considered; other important factors (e.g., age, sex, bone density) were not analyzed. The combined effects of reduced bone mineral density (BMD) and vertebral defects were demonstrated in an experimental study of thoracic cadaver vertebrae subjected to compressive loads after drilling of the centrum to simulate lytic metastases.37 Interestingly, defect size alone did not reliably predict fracture threshold, and BMD was found to be an equally, if not more important, predictive variable. In summary, although vertebroplasty and kyphoplasty are minimally invasive procedures with a low risk of complications, in our experience there are currently no reliable means to predict impeding collapse in asymptomatic patients with vertebral lesions. We have therefore restricted the use of these procedures, at the present time, to patients with axial or mechanical pain that correlates with radiologic findings of vertebral body fracture or collapse. Patients with spinal tumors typically undergo a variety of imaging procedures. MRI must be carefully evaluated for evidence of spinal cord compression or neural foraminal disease caused by tumor or bone/disc fragments. CT is also highly recommended because it best shows the extent of osseous destruction and cortical involvement, particularly any involvement of the posterior vertebral body cortex. Preprocedural plain films are also essential to evaluate the visibility of the bony cortex, and especially the appearance of the pedicles. Because vertebroplasty and kyphoplasty are most commonly performed using fluoroscopy and a transpedicular approach to the vertebral body, poor definition of the pedicles may mandate an alternative approach or the need for needle placement under CT guidance.14 Vertebroplasty is performed under fluoroscopy in a standard angiography suite under sterile conditions, although some authors have emphasized the use of CT for needle positioning or injection assessment.14 All patients have continuous physiologic monitoring with anesthetic support during the procedure. Conscious sedation is sufficient for most patients; however, patients with severe pain may require general anesthesia.8 Patients are positioned prone with horizontal rolls placed under the chest and pelvis. The needle entry site over the pedicle is localized in the anteroposterior plane. After sterile preparation of the skin, a 50/50 mixture of 1% lidocaine and 0.25% bupivacaine is injected with infiltration of the subcutaneous tissue and the periosteum. A 13-gauge needle (Osteo-Site bone biopsy needle, Cook Inc., Bloomington, IN) is introduced through a small dermatotomy and advanced to the posterior aspect of each pedicle along its superolateral cortex. The bevel of the needle is directed so that the tip points laterally, to avoid penetration of the spinal canal.11 The needle is directed anteriorly, medially, and caudally through the pedicle to reach a point within the anterior third of the vertebral body, near the midline in the sagittal plane (Fig. 43-4). Within the vertebral body, the tip is directed medially—the most optimal orientation for PMMA delivery to the vertebral body. If the diagnosis is uncertain, a 16-gauge needle (Franseen biopsy needle, Cook Inc.) may be introduced coaxially at this stage to obtain biopsy samples.38 Once the needle position is confirmed, the bone cement is prepared. We combine 40 mL of the PMMA powder (Simplex P, Stryker-Howmedica-Osteonics, Rutherford, NJ) with 6 g of sterile barium sulfate powder (Bryan, Woburn, MA) for opacification and 1 g of powdered tobramycin (Nebcin, Eli Lilly, Indianapolis, IN) for antibiotic prophylaxis prior to adding the 10 mL of liquid monomer. The preparation is mixed until a doughy, cohesive consistency (similar to toothpaste) is obtained. Multiple 1-mL syringes are filled with the cement mixture. As an alternative, several handheld injection devices are available commercially. Injection is performed under constant fluoroscopic visualization. As the material disperses from the cannula, it fills the interstices of the bone in a three-dimensional pattern (Fig. 43-5). The objective is to fill the anterior two thirds of the vertebral body as visualized on the lateral projection. If PMMA fills less than 50% of the vertebral body, contralateral cannulation and injection may need to be performed. In our experience, significant cross-filling of the vertebral body allows a unilateral approach in 65% of cases.8 Our total injection volumes range from 2 to 8 mL.
Percutaneous Vertebroplasty and Kyphoplasty
 Biomechanical Principles
Biomechanical Principles
 Benign Tumors
Benign Tumors
 Malignant Tumors
Malignant Tumors
 Contraindications
Contraindications
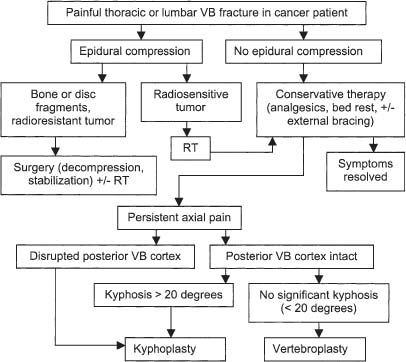
 Impending Collapse
Impending Collapse
 Radiologic Assessment
Radiologic Assessment
 Techniques
Techniques
Vertebroplasty Procedure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree