CHAPTER 166 Peripheral Nerve Stimulation for Neuropathic Pain
Electrical stimulation of peripheral nerves is used in a variety of medical applications. The most common ones include testing neuromuscular conduction in anesthesia and intensive care units1,2; motor stimulation of phrenic nerves in cases of diaphragmal palsy3 and somatic nerves of the extremities in patients with hemiplegia4,5 and paraplegia4,6; vagal nerve stimulation for treatment of intractable epilepsy7 and refractory depression8; autonomic stimulation for urinary9 and gastrointestinal disorders10; and, finally, the stimulation of peripheral nerve for control of neuropathic pain.11
Peripheral nerve stimulation (PNS) has been a part of the neurosurgical armamentarium for a long time. It presents an excellent modality for the treatment of various neuropathic pain conditions and is used to complement the other electrical neuromodulation procedures—spinal cord stimulation (SCS) and deep brain stimulation (DBS). Similar to SCS, PNS is thought to be based on the gate-control theory of pain, suggested by Melzack and Wall more than 40 years ago.12 In terms of approved indications, SCS is by far more accepted than PNS, and frequently PNS is considered a novel technique in the continuum of treatment modalities for medically refractory neuropathic pain. This, however, is a misconception. Although the interest in PNS has been increasing over the past few years, the use of electrical stimulation of the peripheral nerves for pain control is anything but new. When about 40 years ago Wall and Sweet13,14 tried to find a new approach to suppression of neuropathic pain, they inserted an electrode into their own infraorbital foramina and obtained decrease in pain perception during the entire episode of electrical stimulation. Moreover, the first articles dedicated to the idea of PNS with implantable devices (even before the dorsal column stimulation, later known as SCS, was introduced) were published back in 1966 and 1967.13,15 In eight patients with neuropathic pain, the stimulation resulted in lasting pain suppression as long as the stimulator was “on.”13 At about same time, Shelden15 implanted PNS electrodes and stimulated them through an implanted receiver at 14,000 Hz, achieving temporary pain relief.
During the following 20 years, multiple reports appeared in the literature dealing with various applications of PNS, summarizing experience with various types of equipment, and describing different surgical techniques for PNS electrode implantation. The articles that appeared in the 1970s,16–21 1980s,22–28 and 1990s29–32 mostly represented single-institution series with use of different electrodes that were implanted in the vicinity of, or in direct contact with, the peripheral nerve, the same nerve that was thought to be responsible for generation of pain either as a result of direct traumatic or iatrogenic injury or as a part of a complex regional pain syndrome. In most cases, the electrode implantation involved surgical exploration of the peripheral nerve and placement of a flat plate (paddle-type) multicontact electrode immediately next to it. Unfortunately, the reported results of the PNS approach were not extremely encouraging in terms of pain relief. For example, Sweet in 197620 reported an overall long-term success rate of 25%, and in 1982, Nashold and colleagues24 reported successful outcomes in 53% of patients with upper extremity nerve implants and in only 31% of patients with sciatic nerve implantation—a total success rate of less than 43%. In addition, the reports of nerve injury from electrode insertion or stimulation-related fibrosis made PNS less attractive,17,33 particularly because the SCS approach became universally accepted as means of long-term treatment of medically intractable neuropathic pain of various etiologies. Few enthusiastic centers continued using PNS for certain neuropathic pain syndromes, but the lack of wide interest among implanters resulted in little effort from the device manufacturers to get appropriate U.S. Food and Drug Administration (FDA) approval for use of their implantable generators in PNS. Even now, according to the manufacturers’ manuals, the only device specifically approved for peripheral nerve stimulation is a radiofrequency system made by Medtronic (Minneapolis, MN), whereas all other systems, including implantable pulse generators made by Medtronic, as well as devices made by Advanced Neuromodulation Systems (ANS; Plano, TX) and Advanced Bionics (Sylmar, CA), are used for PNS on an off-label basis.
Rebirth of the PNS approach may be credited to pioneering work of Weiner and Reed34 that described the percutaneous technique of electrode insertion in the vicinity of the occipital nerves to treat occipital neuralgia. Soon thereafter, Slavin and Burchiel11,35 described use of this technique in both occipital and trigeminal areas, and afterward, the approach was modified by many implanters in terms of the electrode type, insertion procedure, indications, and other features.36–61
The procedure was not limited to the upper neck and face area; other reports detailed the use of PNS in other parts of the body. For example, percutaneously inserted PNS electrodes were used to control inguinal pain after herniorrhaphy,62 and paraspinal electrodes have been used for treatment of low back pain and sacroiliac pain,63 thoracic postherpetic pain,64 and coccydynia.65
Indications
The usual indications for PNS in neuropathic pain are very similar to those used for SCS procedures. The pain is chronic and severe, negatively affects the patient’s functionality, and is refractory to the usual medical treatment, including medications, physical therapy, and less invasive interventions, such as local anesthetic and sympathetic blocks, application of transcutaneous electrical nerve stimulation (TENS), and injections of botulinum toxin preparations, if these interventions are even considered by the treating physicians. For the purposes of this chapter, the neuropathic pain is broadly defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system,”66 and this wide definition includes, among other syndromes, such debatable nosologic conditions as fibromyalgia, migraines, and coccydynia.
Two other criteria are considered before implantation of a PNS system: the patient’s psychological evaluation and results of a short-term trial with externalized electrodes. Both these steps are taken as the standard approach to SCS; the psychological evaluation details and their predictive values in SCS were recently reviewed.67 Outpatient stimulation trial is also a routine step in SCS surgical procedure, and the trial length usually varies between 2 and 14 days. Usually, a 50% improvement in pain intensity serves as a cutoff limit for considering the trial successful. In some places, the patient satisfaction with pain relief, even if the pain intensity decrease is less than 50%, justifies proceeding with permanent implantation.
In terms of specific indications, there are at least 11 distinct pain conditions for which PNS has been recently reported: (1) postherpetic neuralgia40,45,64; (2) posttraumatic or postsurgical neuropathic pain that is related to underlying dysfunction of a certain nerve, including the infraorbital, supraorbital, or occipital nerves45,48,52; (3) classic migraine, “transformed migraine” presenting with occipital pain and discomfort, and hemicrania continua39,43,46,47,53,54,56,61; (4) occipital neuralgia or cervicogenic occipital pain34,38,47,49,51; (5) complex regional pain syndrome28,30,68; (6) cluster headaches54,58,59,69; (7) chronic daily headaches39,53,60; (8) inguinal pain after herniorrhaphy62; (9) coccydynia65; and (10) fibromyalgia.57,70
Surgical Technique
The hardware for PNS is usually implanted with a straightforward set of guidelines. The electrode (lead) is implanted in the vicinity of the stimulated nerve. The approach may include either direct exposure of the nerve,32 so that the electrode may be placed next to it (or even wrapped around it—similar to vagal nerve stimulation system),7,8,71 or percutaneous electrode implantation through a needle inserted perpendicular to the nerve course.34,48,52,59 Between these two approaches is the technique of surgical lead insertion toward the nerve without actual nerve exposure.44,47,49,58,61
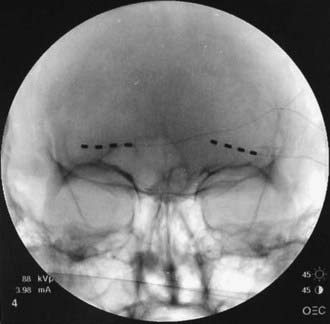
The direction of electrode insertion may be chosen based on implanter’s preference; for example, we routinely insert electrodes from lateral to medial not only in the supraorbital and infraorbital regions (where it is probably the only way to place them) but also in the occipital area,51,52,55 whereas others prefer to insert electrodes from medial to lateral.41,43 Standard 4-, 8- or even 16-contact electrodes61 are used (Figs. 166-1 to 166-3); the electrodes are passed in the epifascial plane under the skin but above the muscles. Our general approach is to have the electrode cross the path of the nerve chosen as a stimulation target. As long as this nerve happens to be either under one of the electrode’s contacts or between the two contacts, the stimulation can be steered toward it to achieve adequate coverage. For the trial insertion, we do not implant any deep anchors or extensions. The electrodes are sutured to the skin with plastic anchors and fine nylon, and a strain-relief loop is created around the insertion site to avoid inadvertent electrode pullout.
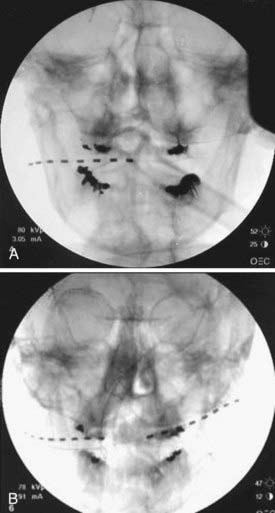
FIGURE 166-1 Anteroposterior radiographs of unilateral (A) and bilateral (B) 8-contact occipital nerve stimulation electrodes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree