FIGURE 46.1 Brachial plexus showing its various constituents and their relationship to structures in the region of the upper chest, axilla, and shoulder.
The three trunks slope laterally and then split into anterior and posterior divisions, from which the three cords are derived. The lower trunk is adjacent to the apex of the lung. The cords of the BP are named for their anatomical relationships to the axillary artery. All the posterior divisions come together to form the posterior cord, which lies posterior to the artery. It is smaller than the other cords and contains little if any contribution from T1. It divides into two major terminal branches: the radial and axillary nerves. The anterior divisions form the medial and lateral cords. The anterior divisions of the upper and middle trunk combine to form the lateral cord, which lies lateral to the artery and terminates in two major branches: the musculocutaneous nerve and the lateral head of the median nerve. The lateral head of the median carries all median sensory functions and the motor innervation to the pronator teres and flexor carpi radialis. The anterior division of the lower trunk continues as the medial cord, which lies medial to the artery, and also terminates in two major branches: the medial head of the median nerve and the ulnar nerve. The medial head of the median nerve carries all of the other median motor functions but has no cutaneous sensory component. After giving off the medial head to the median nerve, the medial cord continues as the ulnar nerve. As a generalization, the posterior cord supplies the extensor muscles, and the lateral and medial cords the flexor muscles.
The roots and trunks of the plexus lie in the posterior triangle of the neck, in the angle between the clavicle and the posteroinferior border of the sternocleidomastoid; the cords lie in the axilla; the divisions span the gap and lie approximately beneath the medial two-thirds of the clavicle, between the clavicle and the first rib. The cords are the longest component of the plexus. In the lower axilla, the BP divides into its terminal branches. The plexus is sometimes divided into a supraclavicular portion (roots and trunks) and an infraclavicular portion (divisions, cords, and terminal branches). In other schemes, the divisions are said to joint the supra-and infraclavicular portions of the plexus. The BP is also broadly divided into the upper plexus (upper trunk and lateral cord) and lower plexus (lower trunk and medial cord). Some pathologic processes have a predilection for different parts of the plexus. Trauma is particularly likely to affect the upper plexus (e.g., Erb’s palsy); lower plexopathies are often non traumatic (e.g., Pancoast tumor or thoracic outlet syndrome). The terminal branches of the BP may be divided into a supraclavicular and an infraclavicular group. The clinically important supraclavicular nerves are the phrenic, long thoracic, suprascapular, and dorsal scapular. The other terminal branches are infraclavicular.
The BP can be involved in a plethora of disease processes. The most common and clinically important of these include neuralgic amyotrophy (NA, acute brachial plexopathy, brachial plexitis, Parsonage-Turner syndrome); trauma, such as with missile and stab wounds or motor vehicle (especially motorcycle) accidents; neoplasms; postradiation plexopathy; obstetrical palsies; postsurgical plexopathy; the “stinger” or “burner” phenomenon that frequently affects football players, which is likely a mild form of plexus injury; and thoracic outlet syndrome.
NA (brachial plexitis, acute BP neuropathy) is a fairly stereotyped clinical syndrome characterized by the acute onset of pain in the shoulder and upper arm, followed by weakness, then atrophy, of variable severity, primarily affecting upper arm and shoulder muscles. The BP can sustain injury in a number of ways: missile and stab wounds, motor vehicle (especially motorcycle) accidents, football, and iatrogenically. Stretch injuries of the plexus occur during childbirth and usually involve the upper plexus (Erb’s palsy), much less often the lower plexus (Klumpke’s palsy) or the entire plexus. Neoplasms, especially breast and lung, may invade the plexus. Radiation plexopathy may complicate treatment of such tumors and appears after a delay of months to years. This is also the time frame in which the radiation therapy may have kept a tumor at bay. Distinguishing recurrent tumor from radiation plexopathy is often difficult.
Other etiologies of brachial plexopathy include external compression (e.g., backpack or rucksack palsy), compression from an internal process (e.g., encroachment on the lower BP from a Pancoast tumor), or involvement in systemic processes such as systemic lupus erythematosus (SLE) or sarcoid, or iatrogenic plexopathy during cardiac surgery. The plexus may rarely be involved in a number of other conditions, including lupus, lymphoma, Ehlers-Danlos syndrome, and infectious or parainfectious disorders. Some of these processes are by nature progressive.
With pressure injuries, the same general rules apply as for other nerves. Mild lesions produce primarily demyelination and can cause severe clinical deficits but have an excellent prognosis. With plexopathies, there may be the additional complication of disease progression. Many of the conditions that affect the plexi are not static. Pancoast tumors continue to grow, radiation damage tends to progress, and systemic diseases such as SLE continue their activity. All these mechanisms of injury make the pathophysiology of plexopathies complex and the clinical evaluation challenging.
The Phrenic Nerve
The phrenic nerve arises from the phrenic nucleus at C3-C5; it also carries some sensory filaments from the diaphragm, pericardium, and pleura. The fibers of the phrenic nerve arise from root level. Unilateral diaphragmatic paralysis is frequently asymptomatic, except for orthopnea and exertional dyspnea. With bilateral paralysis, there is dyspnea on the slightest exertion, a scaphoid abdomen that does not protrude on expiration, absence of Litten’s sign, increased excursion of the costal margins, retraction of the epigastrium on inspiration, overactivity of the accessory respiratory muscles, and difficulty in coughing, sneezing, or making quick forceful inspiratory movements such as sniffing.
The nerve may be involved in NA, damaged by surgical procedures on the neck or chest, or compressed in the mediastinum by enlarged nodes, aneurysms, or neoplasms. The segmental supply to the diaphragm is frequently compromised in upper spinal cord injuries and determines whether the quadriplegic patient will or will not be able to live without a ventilator. Involvement of phrenic motor neurons is common in amyotrophic lateral sclerosis. Other causes of phrenic neuropathy include diabetes mellitus, mediastinal irradiation, sarcoidosis, tuberculosis, Lyme disease, and acute and chronic inflammatory demyelinating polyneuropathies. Idiopathic bilateral phrenic neuropathies causing diaphragmatic paralysis may occur.
The Long Thoracic Nerve
This nerve is derived from the C5-C7 roots and supplies the serratus anterior muscle. Paralysis of the serratus anterior muscle causes winging of the scapula (Figure 27.8). The long thoracic nerve (LTN) may be injured by pressure from carrying heavy objects or packs on the shoulder (backpack or rucksack palsy) or by penetrating wounds. It may be involved in NA, sometimes in isolation. Iatrogenic LTN palsy may follow anesthesia or local invasive procedures on the anterolateral aspect of the thorax. Paralysis may also occur with myopathic processes, such as FSH dystrophy and the scapuloperoneal syndromes.
The Dorsal Scapular Nerve
The dorsal scapular nerve arises directly from the C5 nerve root to innervate the rhomboid muscles. Weakness causes lateral displacement of the vertebral border of the scapula and lateral displacement of the inferior angle. Atrophy may be obscured by the overlying trapezius. Isolated lesions have been reported in bodybuilders. It is occasionally of importance, especially electromyographically, in distinguishing between C5 radiculopathy and upper trunk brachial plexopathy.
The Suprascapular Nerve
This nerve is derived from C5-C6 and arises from the upper trunk. It runs posteriorly through the suprascapular notch, beneath the suprascapular ligament, to innervate the supraspinatus muscle, and then around the glenoid process of the spine of the scapula in the spinoglenoid notch to reach the infraspinous fossa and innervate the infraspinatus. The nerve may be entrapped at the suprascapular notch, causing pain and weakness of both supraspinatus and infraspinatus, or at the spinoglenoid notch, causing weakness of only the infraspinatus. Because of selective fascicular vulnerability, a lesion at the suprascapular notch may also involve only the infraspinatus branch. A common cause of suprascapular neuropathy is NA.
The most common causes of suprascapular neuropathy are occupational overuse, sports-related injury, direct trauma, and ganglion cysts. Suprascapular neuropathy may occur after scapular fracture or by direct pressure (mobile telephone user’s shoulder droop). Repetitive motion injuries in sports that particularly involve strenuous overhead activity pose a particular hazard. The prevalence of infraspinatus muscle atrophy in the hitting shoulders of professional beach volleyball players is 30%.
The Axillary Nerve
The axillary (circumflex) nerve is a terminal branch of the posterior cord of the BP derived from C5-C6. It accompanies the posterior humeral circumflex artery through the quadrangular space and then divides into anterior and posterior branches. The anterior branch supplies the anterior part of the deltoid muscle; the posterior branch supplies the posterior part of the deltoid and the teres minor muscles and sends sensory twigs to a small circular area of skin over the deltoid muscle just above the deltoid attachment. Axillary nerve lesions are usually due to trauma or NA. The nerve may be injured by fracture or dislocation of the humeral head, penetrating wounds, misplaced therapeutic injections, arthroscopy, or direct blows to the shoulder. It is also prone to injury by overhead activity in sports, especially volleyball, tennis, and baseball. There is weakness and wasting of the deltoid, often profound, and a small patch of sensory loss over the shoulder. Isolated lesions of the anterior branch may spare sensation. Conversely, isolated involvement of the sensory branch has been reported after shoulder arthroscopy. Preservation of dorsal scapular and suprascapular nerve function helps distinguish axillary neuropathy from C5 radiculopathy and upper trunk plexopathy, but the evaluation of suprascapular nerve function must usually be made electromyographically, as both the deltoid and supraspinatus are shoulder abductors, and both the teres minor and infraspinatus are external rotators.
The Musculocutaneous Nerve
This nerve is derived from C5-C7 and is a terminal branch of the lateral cord. It passes into the upper arm in the groove between the deltoid and pectoral muscles, sends a branch to the coracobrachialis muscle, then traverses a foramen in the muscle, after which it descends and innervates the biceps and most of the brachialis. At the elbow, it pierces the deep fascia just lateral to the biceps tendon and continues as the lateral antebrachial cutaneous nerve (lateral cutaneous nerve of the forearm) to supply sensation to the lateral aspect of the forearm from the elbow to the thenar eminence. The musculocutaneous may be injured by overly vigorous elbow flexion (weight lifter’s palsy, Figure 46.2). There is weakness of elbow flexion with the forearm supinated and marked weakness of supination. The semipronated forearm can still be flexed by the brachioradialis. There is a relatively small area of sensory loss on the lateral surface of the forearm. The biceps reflex is diminished or absent. Preservation of axillary, dorsal scapular, and suprascapular nerve functions differentiate musculocutaneous palsy from an upper trunk lesion and C5 radiculopathy, and preservation of forearm pronation and lateral hand sensation, median nerve functions, distinguishes from a lateral cord lesion and C6 radiculopathy.
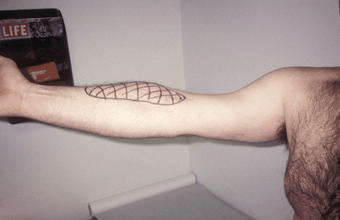
FIGURE 46.2 Musculocutaneous neuropathy after performing onearmed pull-ups. Note the striking biceps atrophy and sensory loss in the distribution of the lateral antebrachial cutaneous nerve.
The Median Nerve
The median nerve has two components: a lateral division and a medial division. The lateral cord of the BP divides into two terminal branches: One becomes the musculocutaneous nerve, and the other becomes the lateral division of the median nerve. The medial cord of the BP also divides into two terminal branches: One forms the medial division of the median nerve, and the other continues as the ulnar nerve. The medial and lateral divisions of the median nerve join to form a single trunk, which passes through the upper arm without branching down to the region of the elbow (Figure 46.3).
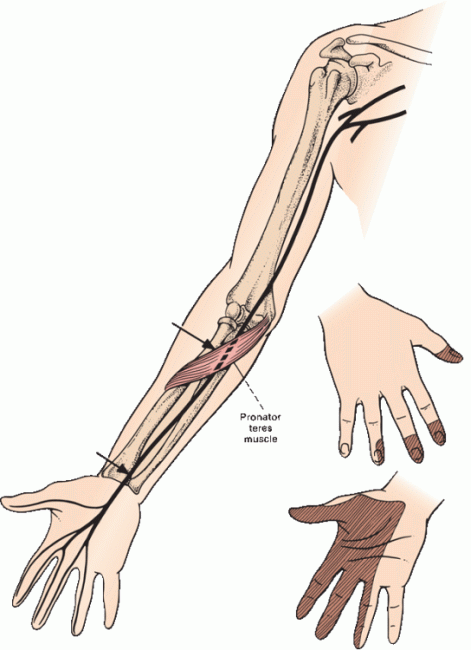
FIGURE 46.3 Common sites for injury to the median nerve and the distribution of sensory loss with a proximal median nerve lesion. In carpal tunnel syndrome (CTS), sensation over the thenar eminence is spared.
There the branches begin to separate. Innervating twigs are given off from the lateral head to the pronator teres and flexor carpi radialis muscles. The main trunk passes through the two heads of the pronator teres muscle and beneath an aponeurosis connecting the two heads of the flexor digitorum superficialis (the sublimis bridge). Just distal to the pronator teres, the nerve gives off the anterior interosseous nerve (AIN), which runs along the interosseous membrane and innervates the median head (lateral portion) of the flexor digitorum profundus (FDP), the flexor pollicis longus, and the pronator quadratus. The AIN has no cutaneous sensory component. The main trunk of the median nerve continues down the forearm, giving off muscular branches to the palmaris longus and flexor digitorum superficialis.
The median nerve crosses from the distal forearm to the hand through the carpal tunnel. The walls and floor of the tunnel are formed by the carpal bones and the roof by the transverse carpal ligament (TCL). The TCL evolves from the antebrachial fascia at about the level of the wrist crease and extends 4 to 6 cm into the palm. The passageway is narrowest 2.0 to 2.5 cm distal to its origin, which corresponds to the usual site of median nerve compression in CTS (Figure 46.4). Lying with the median nerve in the canal are the eight deep and superficial finger flexor tendons and the tendon of the flexor pollicis longus surrounded by a complex synovial sheath.
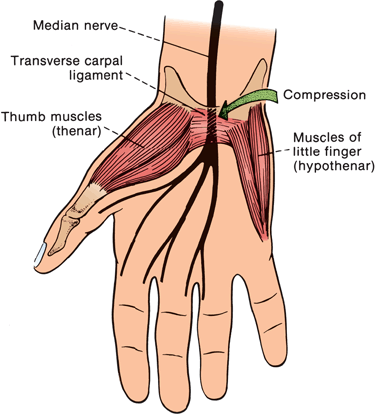
FIGURE 46.4 The relationship of the median nerve to the transverse carpal ligament and the site of compression in CTS.
The palmar cutaneous branch of the median nerve leaves the main trunk 5 to 8 cm proximal to the wrist crease. It travels through its own separate passageway in the TCL and provides sensation to the thenar eminence; it does not traverse the carpal tunnel. Loss of sensation over the thenar eminence is not part of CTS and suggests a lesion proximal to the wrist. After exiting the carpal tunnel, the median nerve gives off its recurrent thenar motor branch, which curves backward and radially to innervate the median thenar muscles (abductor pollicis brevis, opponens pollicis, and lateral head of the flexor pollicis brevis). The nerve ends by giving off terminal motor branches to innervate the first and second lumbricals and then dividing into common digital sensory branches that carry sensory fibers from the palmar surfaces of the thumb, index and middle fingers, palmar aspect of the radial half of the ring finger, and the dorsal aspect of the middle and distal phalanges of the index and middle fingers and radial half of the ring finger. The finger flexor reflex is mediated in part by the median nerve. The pronator reflex, pronation of the forearm after tapping in the region of the radial styloid on the volar surface of the forearm, is also median innervated.
Carpal Tunnel Syndrome
Entrapment of the median nerve beneath the TCL is often brought on or exacerbated by excessive hand/wrist/finger movements; the combination of repetitive finger flexion with wrist motion seems to be the most hazardous ergonomic stress. Both vocational and r ecreational activities can incite or aggravate the condition. Although keyboarding is often blamed, the frequency of CTS in computer users is similar to that in the general population. CTS can rarely result from mass lesions narrowing the passageway (for example, ganglion, osteophyte, lipoma, aneurysm, anomalous muscle). Numerous systemic conditions predispose to CTS, including rheumatoid arthritis, diabetes mellitus, chronic renal insufficiency and hemodialysis, hypothyroidism, amyloidosis, myeloma, acromegaly, and pregnancy. Constriction within the carpal tunnel is often due to nonspecific tenosynovitis of the flexor tendons. A congenitally narrow canal may predispose some patients.
CTS produces a characteristic clinical picture of hand pain, numbness, and paresthesias, all usually more severe at night. Patients often claim relief by shaking or flicking (see below) the hand. The reason for the nocturnal exacerbation of symptoms remains obscure, but the diagnosis should remain suspect in the absence of this feature. Proximal upper-extremity pain, usually in the forearm but sometimes as far as the shoulder, is less typical but not uncommon. Many patients complain of “whole hand” numbness, and rarely, for unclear reasons, a patient with CTS may present with ulnar or even radial distribution paresthesias. In a survey of 100 patients with elecro-diagnostically confirmed CTS and no other pathology, symptoms were most commonly reported in both median and ulnar digits, followed by median digits only and a glove distribution. Unusual sensory patterns were reported by some patients. In another study, over 50% of patients with exclusive CTS had tingling or numbness involving the whole hand, ulnar or radial nerve distributions. Some patients reported symptoms proximal to the wrist.
Findings on examination vary with the severity of the condition. Patients with mild CTS may have a normal physical exam or trivial sensory loss over the fingertips. The earliest sensory loss seems to occur over the volar tip of the middle finger. Patients with more advanced disease have more easily demonstrable sensory loss and frequently have weakness of the thenar muscles. The opponens pollicis is occasionally, and the abductor pollicis brevis rarely, innervated by the ulnar nerve and may be spared in some patients. The lumbrical muscles are usually spared. Although there may be sensory complaints in unusual distributions, sensory signs do not extend beyond the median nerve territory, recalling of course that there may be variations in the territory of cutaneous nerves (Figure 36.5)
Patients with severe involvement demonstrate thenar weakness and atrophy and dense sensory loss. Tinel’s sign is paresthesias produced by percussion over a peripheral nerve that may indicate focal nerve pathology. Eliciting a Tinel’s sign can be useful, but many normal patients “Tinel” over all their nerves; only the presence of a disproportionately active Tinel’s sign over the clinically suspect nerve has any localizing value. Phalen’s (wrist flexion) test is numbness or paresthesias in the median distribution produced by forceful flexion of the wrist for 1 minute. The reverse Phalen’s (prayer) test is the same but with the wrist hyperextended. In the carpal compression maneuver, the examiner applies firm thumb pressure over the median nerve at the wrist crease, seeking to reproduce CTS symptoms. These provocative tests have proven disappointing, with high proportions of false positives and false negatives. The “flick” sign, in which the patients flick the wrist to demonstrate what they do to “restore the circulation” at night is more useful but still imperfect. The rare “reverse Tinel’s sign” with paresthesias radiating retrograde up the forearm may be more specific for CTS. The tourniquet test (cuff compression test) seeks reproduction of pain and paresthesias with compression above systolic pressure. The elevated arm stress test (Roos’ test) has been touted as useful in both thoracic outlet syndrome and CTS but has a high incidence of false positives in both.
The most common differential diagnostic exercise is between CTS and cervical radiculopathy, most often C6. Neck and shoulder pain, weakness in C6 innervated muscles, reflex changes, sensory loss restricted to the thumb, the absence of nocturnal paresthesias, and reproduction of the paresthesias with root compression maneuvers all favor cervical radiculopathy. Other conditions occasionally meriting consideration include proximal median neuropathy, neurogenic thoracic outlet syndrome, and upper brachial plexopathy. Various musculoskeletal conditions, especially de Quervain’s tendonitis, can cause hand and wrist pain suggestive of CTS.
Proximal Median Neuropathy
The proximal median nerve is well protected by soft tissues and, accordingly, is injured less frequently than either the radial or the ulnar nerve. It may be involved in dislocations of the shoulder, injuries of the elbow joint, fractures of the humerus or radius, penetrating wounds, or compression injuries. Proximal median neuropathy may be a complication of shunt placement for hemodialysis. A triad neuropathy is involvement of median, ulnar, and radial nerves, usually from a lesion in the axilla, for example, crutch palsy, or of the BP distal branches. A complete proximal median nerve lesion causes paralysis of flexion of the wrist and radial fingers, forearm pronation, and thumb abduction, opposition, and flexion. Finger flexion at the metacarpophalangeal (MCP) joints may be partially preserved because of preserved interosseous function. Loss of ability to flex the distal phalanx of the index finger, without a bone or tendon lesion to account for it, is pathognomonic. The thumb lies adducted and extended; it cannot be opposed to the tip of the little finger or abducted at right angles to the palm (palmar abduction), and the terminal phalanx cannot be flexed (Figure 46.5). Many of the lost movements, except for flexion of the distal phalanx of the index finger and movements of the thumb, can be substituted for by ulnar innervated muscles. There is no substitution for palmar abduction, and comparison of this movement on the two sides is an important test of median nerve function. Thenar atrophy with the thumb rotated and resting in adduction produces the simian (ape) hand deformity (“monkey paw”). Loss of finger flexion when attempting to make a fist produces a posture resembling the hand used by clergy in making a benediction, a term best avoided (see below).
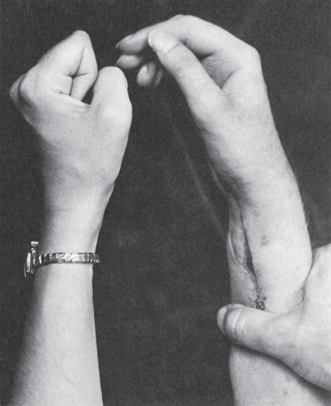
FIGURE 46.5 Motor changes secondary to a lesion of the median nerve, showing loss of flexion of the distal phalanges of the radial fingers.
The sensory changes involve the radial side of the palm, including the thenar region (palmar cutaneous distribution), the index and middle fingers, and the radial half of the ring finger. They are less complete on the dorsum of the hand than on the palmar surface, and usually involve only the distal (or middle and distal) phalanges of the index and middle fingers, and sometimes part of the thumb and radial half of the ring finger (Figure 36.4). There are no significant reflex changes. Median nerve paralysis is often accompanied by vasomotor and trophic changes and by intractable, burning pain (causalgia, reflex sympathetic dystrophy, complex regional pain syndrome) especially if the lesion is incomplete. The skin may be flushed, cyanotic, and either wet or dry; the nails are brittle or striated, and there may be changes in hair growth.
The median nerve may rarely be entrapped by the ligament of Struthers, an anomalous fibrous band running from a distal humeral supracondylar spur to the medial epicondyle (ME). In the pronator teres syndrome, the median nerve is entrapped at the point where it passes through the two heads of the pronator teres; it may affect the main trunk, causing both motor and sensory dysfunction (Seyffrath’s syndrome); more often, only the AIN is involved (Kiloh-Nevin syndrome). Hypertrophy of the pronator teres has been implicated. There is often pain in the proximal forearm, and there may be tenderness and/or a Tinel’s sign over the pronator muscle. Depending on the individual anatomy and the origin of the branch to the pronator teres, the pronator teres may or may not be involved in a pronator syndrome. The pronator quadratus is involved with either proximal median neuropathy or the AIN syndrome; distinguishing between pronator teres and pronator quadratus requires careful elbow positioning (see Chapter 27).
Complete AIN paralysis causes inability to flex the distal phalanx of either the thumb or index finger. The patient cannot make a circle by touching the tip of the thumb to the tip of the index finger, making a triangle instead by touching the finger pads (pinch sign, or OK sign [the patient is unable to make the OK sign with the involved hand]) (Figure 46.6). There are no cutaneous sensory changes in AIN palsy, but there may be pain carried by afferent fibers innervating joints. AIN palsy often occurs as an isolated manifestation of NA. Other etiologies include strenuous exertion, especially when involving flexion and pronation of the forearm, trauma, for example, fracture, venipuncture, penetrating injury, and intraoperative positioning. In the pseudo-AIN syndrome, the lesion predominantly involves AIN fascicles in the main trunk of the median nerve. There may be median distribution sensory changes. The usual etiology is a supracondylar fracture. Median neuropathy proximal to the carpal tunnel may occur in wheelchair athletes.

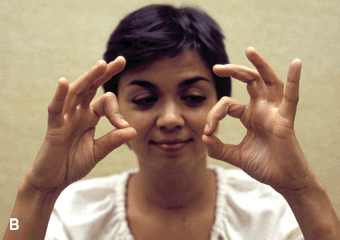
FIGURE 46.6 Anterior interosseous neuropathy; the patient is unable to flex the distal phalanx of the thumb or index finger and is thus unable to make the “OK sign.” A. Patient on the left, control on the right. B. Involved side is the patient’s right.
The Ulnar Nerve
The ulnar nerve arises as a continuation of the medial cord of the BP. As it exits from the thorax, it passes through the axilla and into the upper arm lying medial to the brachial artery in a common neurovascular sheath with the median nerve and the medial brachial and antebrachial cutaneous nerves. At about the level of the insertion of the coracobra-chialis, the ulnar leaves the common neurovascular bundle and pierces the medial intermuscular septum to gain the posterior compartment of the arm. The nerve then descends toward the elbow in a groove alongside the medial head of the triceps. The point of the ulnar nerve’s penetration of the medial inter-muscular septum and the nearby deep fascia binding the nerve in the triceps groove are sometimes referred to as the arcade of Struthers, a potential entrapment site (not to be confused with the ligament of Struthers). Whether the arcade of Struthers actually exists remains a point of disagreement. After piercing the medial intermuscular septum, the nerve slants distally and medially, and then traverses the retroepicondylar (ulnar) groove between the ME and olecranon process (OP). It then passes beneath the humeroulnar aponeurotic arcade (HUA), a dense aponeurosis joining the humeral and ulnar heads of origin of the flexor carpi ulnaris (FCU) muscle, which typically lies 1.0 to 2.5 cm distal to a line connecting the ME and the OP.
After passing under the HUA, the nerve runs through the belly of the FCU, then exits through the deep flexor-pronator aponeurosis lining the deep surface of the muscle 4.0 to 6.0 cm beyond the ME, and then runs distally toward the wrist. The ulnar palmar cutaneous branch arises in the mid to distal forearm and pursues a separate course to the hand. It enters the hand superficial to Guyon’s canal and supplies sensation to the skin of the hypothenar region. The large dorsal ulnar cutaneous (DUC) branch leaves the main trunk 5 to 10 cm proximal to the wrist to wind posteriorly and emerge on the dorsal surface of the wrist to provide sensation to the dorsal, ulnar aspect of the hand, as well as the small and ring fingers.
The ulnar nerve enters the hand through Guyon’s canal. The TCL, which forms the roof of the carpal tunnel, dips downward as it spans medially and forms the floor of Guyon’s canal. The pisohamate ligament, which runs from the pisiform bone to the hook of the hamate, forms the distal part of the floor of the canal. The volar carpal ligament, a thin investment that is basically a continuation of the deep forearm fascia arches over and forms the roof of Guyon’s canal along with the thin palmaris brevis muscle. The hook of the hamate forms the lateral, and the pisiform bone and FCU tendon the medial, boundaries.
As it emerges from beneath the volar carpal ligament, the ulnar gives a branch to the palmaris brevis and then branches into the superficial terminal sensory division and the deep palmar division. The deep branch exits Guyon’s canal, passes through the pisohamate hiatus, and then arches laterally beneath the flexor tendons, innervating the interossei and breaking up into terminal branches on reaching the adductor pollicis and first dorsal interosseous. The deep head of the flexor pollicis brevis is usually supplied by a short twig from the terminal branch to the adductor pollicis.
Ulnar Neuropathy at the Elbow
UNE is most often due to compression in the retro-epicondylar groove but may be due to entrapment beneath the HUA, other entrapment sites are rare. UNE was originally described in patients with elbow deformities due to remote fracture and/or dislocation. The UNE occurred because of chronic compression and stretch and typically followed the injury by months or years (tardy ulnar palsy). Gradually, the term tardy ulnar palsy became a generic for any UNE, even without a history or evidence of elbow joint pathology. Compression at the HUA was actually recognized in the 1920s by Buzzard and Sargent, but it was not until the landmark Canadian papers of the 1950s that it became widely known. Fiendel and Stratford proposed the term cubital tunnel syndrome (cubit is Latin for elbow) to refer to compression by the HUA. The title of their paper is telling: “The role of the cubital tunnel in tardy ulnar palsy.” Gradually, cubital tunnel syndrome has replaced tardy ulnar palsy as a generic referring to any UNE. The term is thus used very inconsistently, and has outlived its usefulness, but is very entrenched. Although rare cases of UNE are caused by ganglia, tumors, fibrous bands, or accessory muscles, most are caused by external compression, repeated trauma or repetitive elbow flexion. Chronic minor trauma and compression, including leaning on the elbow, can result in UNE at the groove. It can also occur in patients who suffer compression during anesthesia or coma.
In the majority of patients with UNE, the initial symptoms are intermittent numbness and tingling in the ulnar nerve distribution, often associated with elbow flexion. Occasionally, the initial problems may be motor dysfunction, such as a feeling of weakness of grasp and pinch, or a loss of dexterity. Patients may not see a physician until the initially small degrees of intrinsic muscle atrophy become difficult to overlook. A history of elbow fracture or dislocation, acute blunt trauma, chronic occupational trauma, or arthritis may be important. When there is no relevant history, entrapment at the HUA should be considered. An early motor symptom sometimes noted is loss of control of the small finger, which may cause the finger to get caught when the patient is trying to place the hand in a pocket, and examination may show an abducted posture of the small finger (Wartenberg’s sign), both due to weakness of the third palmar interosseous muscle.
Examination usually discloses weakness of ulnar innervated hand intrinsics. Not all intrinsics are necessarily involved to an equal degree; the first dorsal interosseous is the most commonly affected. Weakness of the adductor pollicis interferes with thumb adduction; impaired adduction is often tested by trying to elicit Froment’s sign. The patient is asked to hold a piece of paper between palm and thumb, and the examiner attempts to withdraw it. With weak thumb adduction, the patient will substitute the flexor pollicis longus and flex the IP joint of the thumb. Weakness of the FCU and/or the FDP to the ring and small fingers reliably point to an elbow lesion. However, the ulnar forearm muscles are frequently spared in UNE, so the lack of clinical, or even electromyographic, abnormality in these muscles in no way excludes a lesion at the elbow. Non-ulnar innervated hand and forearm muscles should be systematically assessed in suspected ulnar neuropathy. Weakness of non-ulnar muscles is the usual clue to disease involving the lower BP or C8 root. In the elbow flexion test, the elbow is held fully flexed and pressure applied just distal to the ulnar groove to elicit paresthesias. A variant is to hold the elbow flexed and the wrist flexed in ulnar deviation.
The lumbricals flex the MCP joints and extend the interphalangeal (IP) joints. The lumbricals for the ring and small fingers are normally supplied by the ulnar nerve and those for the index and middle fingers by the median. In ulnar lesions, unopposed extensor tone at the fourth and fifth MCP joints and unopposed flexor tone at the IP joints produce the ulnar griffe or claw deformity (Figure 46.7). Clawing varies, depending upon the amount of muscle weakness, the laxity of the MCP joints, and the level of the lesion. A “low” (distal) ulnar lesion with preserved function of the FDP induces more clawing than a “high” (proximal) ulnar lesion, where the accompanying FDP weakness creates less of the unopposed flexor pull deforming the ring and small fingers. The term benediction hand (hand of the papal benediction, papal hand) is sometimes used to refer to an ulnar griffe with the hand at rest and sometimes to a high median neuropathy when the patient is attempting to make a fist. The hand posture is somewhat similar in that the ring and small fingers are flexed and the index and middle fingers are not. Usage favors median neuropathy in the neurology literature and ulnar neuropathy in the nonneurology literature. There is conjecture that a medieval pope had this hand deformity, and his successor learned it as the proper hand position for blessing the masses, passing it down as tradition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







