FIGURE 16.1 Components of the facial nerve in the pons. (Modified from Kiernan JA. Barr’s The Human Nervous System: An Anatomical Viewpoint. 9th ed. Philadelphia: Wolters Kluwer/Lippincott, Williams & Wilkins, 2009, with permission.)
CN VII exits the pons laterally at the pontomedullary junction, just caudal to the roots of CN V between the olive and the inferior cerebellar peduncle (Figure 11.3). The NI is a small bundle that usually leaves the pons closer to CN VIII than CN VII and runs between the larger trunks across the cerebellopontine angle (CPA). In about 20% of specimens, the NI is not identifiable as a separate structure in the CPA. At the entrance to the IAC, the facial nerve motor root lies in a groove on the anterosuperior surface of the vestibulocochlear nerve, with the NI in between. In this segment, CN VII is a paler white color than CN VIII. The facial nerve at this point lies in close proximity to the anterior inferior cerebellar artery (AICA). In some individuals, the AICA loops down into the IAC. As with the vaginal sheaths of the optic nerve, the subarachnoid space extends along the facial nerve to the geniculate ganglion.
At the bottom or lateral end of the IAC, the nerve pierces the meninges and enters the facial canal, or fallopian aqueduct. The point of entry is the narrowest portion of the canal. The facial nerve and the NI merge as the nerve enters the canal. In traversing the facial canal, the nerve makes two abrupt, tortuous turns, creating two external genus. In its course through the petrous bone, from its entrance into the facial canal until its exit from the stylomastoid foramen, the nerve has three segments: labyrinthine, horizontal or tympanic, and mastoid or vertical. The labyrinthine segment lies laterally between the cochlea and vestibule, toward the medial wall of the tympanic cavity, running perpendicularly to the long axis of the petrous pyramid. The labyrinthine segment ends at the first external genu where the geniculate ganglion lies. At this point, the nerve turns abruptly and runs horizontally for about 1 cm (the horizontal or tympanic segment), then turns backward and arches downward behind the tympanic cavity (mastoid or vertical) segment. The branch to the stapedius muscle arises from the distal tympanic or upper end of the mastoid segment. At the end of the tympanic segment, the nerve encounters the second external genu as it makes a 90-degree turn to enter the mastoid segment. The mastoid segment then descends toward the stylomastoid foramen, gives off the chorda tympani about 6 mm before its exit, and emerges from the stylomastoid foramen. The tight confines of the bony canal may make the nerve particularly vulnerable to damage from inflammation and edema, a point of possible significance in some CN VII neuropathies (see below). In patients with Bell’s palsy, the involved side usually correlates with the side of the narrower facial canal as determined by high-resolution computed tomography (CT). CN VII runs along with the labyrinthine branch of the AICA, but there is evidence to suggest it is less well vascularized in its intrapetrous segment, particularly in the labyrinthine segment, than elsewhere along its course. This may also have relevance to the pathologic changes in Bell’s palsy.
There may be anatomical variations in the nerve’s course through the petrous bone. It may split into two or three strands at or distal to the geniculate ganglion. The more proximal the division into strands, the more bizarre the subsequent course. Facial motor fibers may run in an enlarged chorda tympani, diminishing the distal facial nerve into a tenuous strand exiting through a narrowed stylomastoid foramen.
Just after exit, the posterior auricular, digastric, and stylohyoid branches arise. The posterior auricular branch supplies the occipitalis, posterior auricular, and transverse and oblique auricular muscles. The digastric and stylohyoid branches supply respectively the posterior belly of the digastric and the stylohyoid. The nerve turns forward and passes into the parotid gland. Within the substance of the parotid, it divides into temporofacial and cervicofacial divisions at the pes anserinus (intraparotid plexus) in the cleft between the superficial and deep lobes of the gland (Figure 16.2). The temporofacial branch crosses the zygoma about 1 cm anterior to the ear, where it is vulnerable to injury.
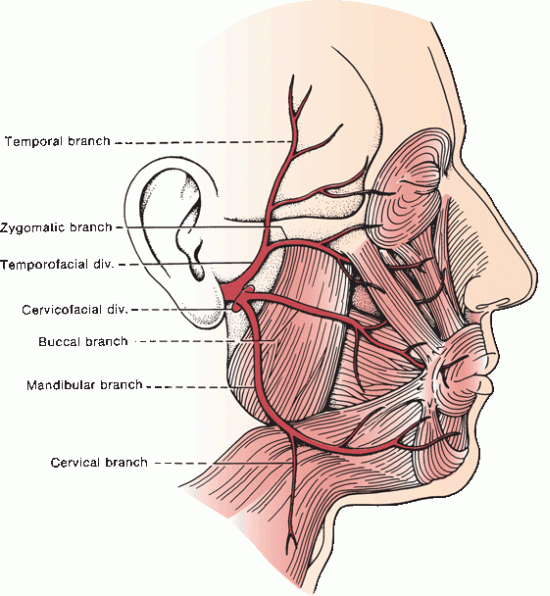
FIGURE 16.2 Branches and distribution of the facial nerve.
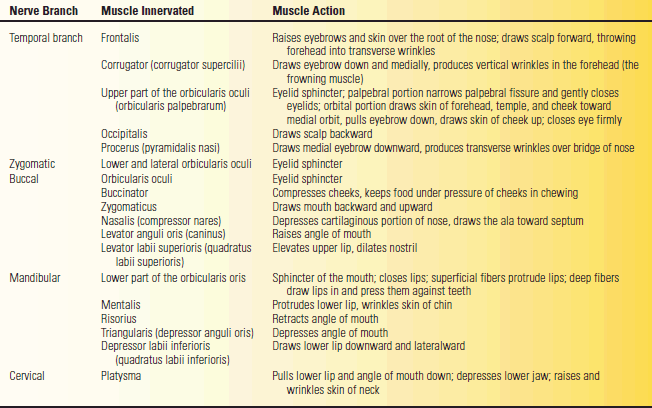
The facial nerve supplies all the muscles of facial expression from the scalp and forehead through the platysma, including the extrinsic and intrinsic muscles of the ear. The muscles of facial expression are responsible for all voluntary and involuntary movements of the face except those associated with movement of the jaws, and for all play of emotions upon the face. The muscles innervated by the terminal branches are summarized in Table 16.1.
TABLE 16.1 Muscles of the Face, Their Actions, and Innervations
The Nervus Intermedius
The NI is the sensory and autonomic component of the facial nerve. It runs in a position intermediate between CNs VII and VIII across the CPA, moving ever closer to the main facial nerve trunk as it enters the facial canal. At the external first external genu, the NI fuses with the geniculate ganglion. The sensory cells located in the geniculate ganglion are general somatic afferent (GSA) and special visceral afferent (SVA). The GSA fibers carry exteroceptive impulses from the region of the external auditory canal and tympanic membrane. The SVA fibers convey taste from the anterior two-thirds of the tongue. The autonomic component of the NI consists of preganglionic general visceral efferent parasympathetic fibers from the superior salivatory and lacrimal nuclei, which consist of scattered cells in the reticular formation near the caudal end of the motor nucleus. Their axons are bound for the submandibular gland enroute to the sublingual and submaxillary glands, the lacrimal glands, and glands in the nasal mucosa.
Course and Branches of the Facial Nerve
The first branch given off in the facial nerve’s course is the greater (superficial) petrosal nerve, which carries preganglionic parasympathetic fibers (Figure 16.3). These fibers are conveyed by the NI to the geniculate ganglion. They pass through the ganglion without synapsing into the greater petrosal nerve, which goes forward through the hiatus of the facial canal to join the deep petrosal nerve from the carotid sympathetic plexus to form the vidian nerve, or the nerve of the pterygoid canal, which runs to the sphenopalatine ganglion, from where postganglionic fibers proceed to the lacrimal gland.
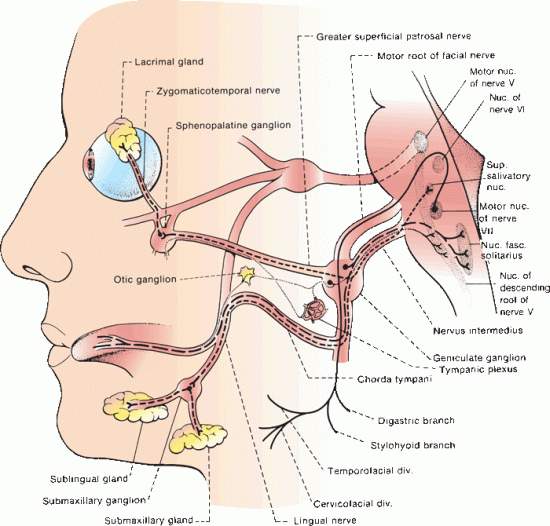
FIGURE 16.3 Course and branches of the facial nerve.
Distal to the geniculate ganglion, the facial nerve continues to descend. As above, the nerve to the stapedius arises from the distal tympanic or upper mastoid segment and passes forwards through a small canal to reach the muscle. Although there is some variability, the chorda tympani usually leaves the main trunk slightly above the stylomastoid foramen; it carries taste and general visceral afferent (GVA) fibers as well as preganglionic parasympathetics. It runs forward and upward in a minute canal in the posterior wall of the tympanic cavity, acquires a mucous membrane investment, and then enters and crosses the middle ear. It is sometimes visible as a small white cord behind the tympanic membrane on otoscopic examination. The chorda tympani runs downward and forward to exit the skull and join the lingual nerve, a branch of the mandibular division of CN V, on its posterior border.
Fibers carrying somatosensory afferents in the chorda tympani have their cell bodies in the geniculate ganglion. The peripheral processes innervate part of the external auditory canal, the tympanic membrane, lateral surface of the pinna, and a small area behind the ear and over the mastoid process. There is a marked individual variation in this distribution. Their central processes terminate in the spinal tract and nucleus of the trigeminal, and the central connections are identical with those of the trigeminal nerve. CN VII may also subserve deep pain and deep pressure from the face.
Taste sensation from the anterior two-thirds of the tongue is carried through the lingual nerve to the chorda tympani, then to the geniculate ganglion. CN VII may also carry taste sensation from the mucosa of the soft palate through the sphenopalatine ganglion. Central processes carrying taste and GVA sensation terminate in the nucleus of the solitary tract. The solitary tract sends communications to the superior and inferior salivatory nuclei, which send parasympathetics to the salivary glands. Other fibers synapse in the reticular formation; next order neurons form a component of the reticulospinal tract bilaterally to synapse with sympathetic neurons in the intermediolateral gray column of the upper thoracic spinal cord. These send sympathetic innervation via the superior cervical ganglion to the salivary glands. Fibers subserving taste sensation ascend with the contralateral medial lemniscus to the thalamus. The primary gustatory cortex, located in the anterior insula and the frontal operculum mediates the perception of taste. Taste fibers also communicate with the hypothalamus and the olfactory system.
The chorda tympani also carries preganglionic parasympathetic fibers to the submandibular ganglion. Postganglionic fibers convey secretory and vasodilator impulses to the submandibular and sublingual salivary glands and mucous membranes of the mouth and tongue (Figure 16.3). These glands also receive sympathetic innervation through the superior cervical ganglion and the carotid plexus. The parasympathetic fibers cause vasodilation and a copious, thin, watery secretion high in enzymes; the sympathetic fibers cause vasoconstriction and a scant, thick, mucoid secretion low in enzyme content.
CLINICAL EXAMINATION
Examination of the Motor Functions
Examination of facial nerve motor functions centers on assessment of the actions of the muscles of facial expression. A great deal can be learned from simple inspection. At rest the face is generally symmetric, at least in young individuals. With aging, the development of character lines may cause asymmetry that does not indicate disease. Distinguishing minor, clinically insignificant, facial asymmetry from subtle facial weakness is sometimes challenging. Note the tone of the muscles of facial expression, and look for atrophy and fasciculations. Note the resting position of the face and whether there are any abnormal muscle contractions. Note the pattern of spontaneous blinking for frequency and symmetry. A patient with parkinsonism may have infrequent blinking and an immobile, expressionless, “masked” face. Facial dystonia causes an abnormal fixed contraction of a part of the face, often imparting a curious facial expression. Progressive supranuclear palsy may cause a characteristic facial dystonia with knitting of the brows and widening of the palpebral fissures (omega sign). Synkinesias are abnormal contractions of the face, often subtle, synchronous with blinking or mouth movements; they suggest remote facial nerve palsy with aberrant regeneration. Spontaneous contraction of the face may be due to hemifacial spasm (HFS). Other types of abnormal involuntary movements that may affect the facial muscles include tremors, tics, myoclonic jerks, chorea, and athetosis (see below).
Observe the nasolabial folds for depth and symmetry and note whether there is any asymmetry in forehead wrinkling or in the width of the palpebral fissures with the face at rest. A flattened nasolabial fold with symmetric forehead wrinkles suggests a central (upper motor neuron) facial palsy; a flattened nasolabial fold with smoothing of the forehead wrinkles on the same side suggests a peripheral (lower motor neuron) facial nerve palsy. Eyelid position and the width of the palpebral fissures often provide subtle but important clinical clues. Eyelid position is discussed further in Chapter 14. A unilaterally widened palpebral fissure suggests a facial nerve lesion causing loss of tone in the orbicularis oculi muscle, the eye closing sphincter; this is sometimes confused with ptosis of the opposite eye. It is a common misconception that facial nerve palsy causes ptosis.
Some diseases cause a characteristic abnormality of facial expression that can sometimes be recognized at a glance, either because of facial immobility or some peculiar facial expression. Examples of primarily neurologic conditions include parkinsonism and related extrapyramidal disorders (masked facies), progressive supranuclear palsy (facial dystonia, omega sign), Möbius’ syndrome, myotonic dystrophy (hatchet face, myopathic face), facioscapulohumeral muscular dystrophy (myopathic face, transverse smile), general paresis (facies paralytica), myasthenia gravis (myasthenic snarl), facial nerve palsy (unilateral or bilateral), and Wilson’s disease (risus sardonicus). These are discussed in the sections dealing with these particular diseases. There are of course numerous congenital syndromes that cause distinctively dysmorphic facies.
Observe the movements during spontaneous facial expression as the patient talks, smiles, or frowns. Certain upper motor neuron facial palsies are more apparent during spontaneous smiling than when the patient is asked to smile or show the teeth. In infants, facial movements are observed during crying. Have the patient grin, vigorously drawing back the angles of the mouth and baring the teeth. Note the symmetry of the expression, how many teeth are seen on each side and the relative amplitude and velocity of the lower facial contraction. Have the patient close her eyes tightly and note the symmetry of the upper facial contraction. How completely the patient buries the eyelashes on the two sides is a sensitive indicator of orbicularis oculi strength.
Other useful movements include having the patient raise the eyebrows, singly or in unison, and noting the excursion of the brow and the degree of forehead wrinkling; close each eye in turn; corrugate the brow; puff out the cheeks; frown; pucker; whistle; alternately smile and pucker; contract the chin muscles; and pull the corners of the mouth down in an exaggerated frown to activate the platysma. There is no good command for platysma contraction, and the movement must be demonstrated. The platysma can also be activated by having the patient open the mouth against resistance or clinch the teeth. The patient may smile spontaneously after attempting to whistle, or the examiner may make an amusing comment to assess emotional facial movement. Because of their paucity of facial expression, patients with Parkinson’s disease may fail to smile after being asked to whistle: the whistle-smile (Hanes) sign.
Trying to gently push down the uplifted eyebrow may detect mild weakness. It is difficult to pry open the tightly shut orbicularis oculi in the absence of weakness. Vigorously pulling with the thumbs may sometimes crack open a normal eye. If the examiner can force the eye open with her small fingers, then the orbicularis oculi is definitely weak. Likewise, it is difficult to force open the tightly pursed lips in a normal individual. When the orbicularis oris sphincter is impaired, the examiner may be able to force air out of the puffed cheek through the weakened lips. Testing ear and scalp movements is seldom useful, although loss of the ability to wiggle the ear in someone previously able to do so has been cited as a sensitive sign of peripheral facial palsy (PFP). The stylohyoid muscle and posterior belly of the digastric cannot be adequately tested. With stapedius weakness, the patient may complain of hyperacusis, especially for low tones. Other tests of motor function and confirmatory signs of facial paresis are discussed in the following sections. It is important in patients with PFP to examine the ear for vesicles or rash, indicative of zoster infection, and to palpate the parotid to exclude a mass lesion.
Examination of the Reflexes
The corneal and other reflexes mediated largely by CN V are discussed in Chapter 15. Frontal release signs such as the snout, suck, and palmomental reflexes are discussed in Chapter 40. Various other reflexes mediated in large part by CN VII can be obtained, but are of little practical value. Some merit brief discussion. They are summarized in Table 16.2.
TABLE 16.2 Facial Reflexes
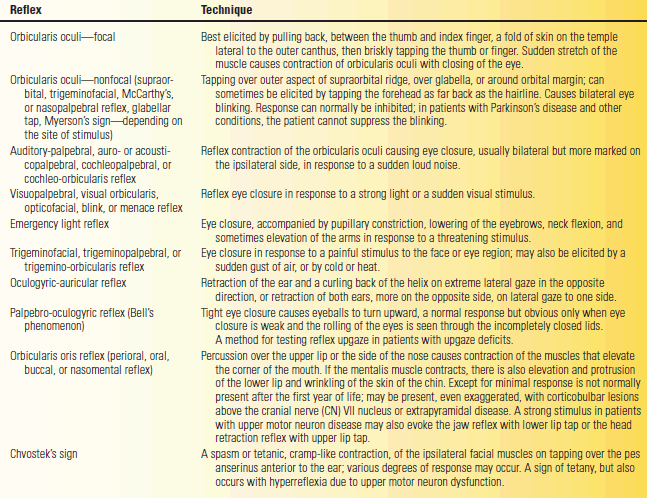
Wartenberg wrote at length about the orbicularis oculi reflex, which he considered an important reflex, and the “chaos of nomenclature concerned with this reflex.” A reflex contraction of the orbicularis oculi causing an eye blink—the nonfocal orbicularis oculi reflex—can be elicited in different ways. The threshold for reflex contraction is very low and the reaction very quick. Tapping with a finger or percussing with a reflex hammer at many different sites over the forehead and about the eyes may elicit a reflex eye blink. Wartenberg said the muscle “reacts … easily to … a multitude of external stimuli.” Different names were given to methods of eliciting the reflex by stimulating different areas, all essentially the same response. The most frequently used version currently is the glabellar tap. Patients with Parkinson’s disease are unable to inhibit the reflexive eye blinks (Myerson’s sign; not to be confused with Myerson’s reflex). Despite the widespread use of the eponym, it is in fact difficult to find any clear reference linking Myerson to the glabellar tap reflex.
A more specific orbicularis oculi reflex is the focal “deep muscle” response elicited from one side by a percussion that stretches the muscle. A fold of the muscle at the temple is held between the thumb and forefinger and then percussed to stretch it back toward the ear. Wartenberg thought this reflex useful because it may be decreased in PFP in proportion to the severity of the palsy, but it is normal or increased with facial weakness of central origin.
Examination of the Sensory Functions
Testing of CN VII sensory functions is limited to taste. Although Hitselberg described hypesthesia of the posterior wall of the external auditory meatus in proximal facial nerve lesions, there is no reliable way to assess the small sensory contribution the nerve makes to the skin of the external ear region. The peripheral receptors are the taste buds embedded in the tongue epithelium, and to a lesser extent in the soft palate and epiglottis. Taste buds respond preferentially, but not solely, to one taste quality. Taste is also carried through CN IX and probably CN X.
There are five primary tastes: bitter, sour, sweet, salty, and umami (delicious or savory). Umami has only recently been added to the list. It is a response to compounds of some amino acids, particularly l-glutamate. Umami is a Japanese term that has no English translation. The many flavors encountered in life are a combination of the primary tastes plus olfaction and oral sensory information (“mouth feel”). Sweet and salty substances are most commonly employed for clinical bedside testing due to their ready availability; sour and bitter are more difficult to come by. Chemosensory referral centers typically use four substances for testing: sucrose (sweet), sodium chloride (salty), quinine (bitter), and citric acid (sour). CN VII only subserves taste on the anterior two-thirds of the tongue. When the tongue is retracted into the mouth, there is rapid dispersion of the test substance outside the area of interest. The tongue must therefore remain protruded throughout testing of an individual substance, and the mouth must be rinsed between tests. If bitter is tested, it should be last because it leaves the most aftertaste.
Some examiners prefer to manually hold the patient’s tongue with a piece of gauze to prevent retraction. Since the patient will be unable to speak with the tongue protruded, instructions must be clear in advance. The patient may raise the hand using some signaling system when taste is perceived, point to words written on paper, or make a similar nonverbal response. A damp applicator stick may be dipped into a packet of sugar, artificial sweetener, or salt and coated with the test substance and then placed on one side of the patient’s tongue and rubbed around. The patient signals whether she can identify the substance. Most patients will identify the test substance in less than 10 seconds. Taste sensation is less on the tip of the tongue, and the substance is best applied to the dorsal surface at about the junction of the anterior and middle third of the tongue. The sweetness of artificial sweeteners such as saccharine and aspartame is more intense, and they may make better test substances than ordinary sugar. For a demonstration of taste testing technique see http://www.youtube.com/watch?v=ldkpd88KSUA&feature=mfu_in_ order&list=UL. More sophisticated methods are available to test for subtle dysfunction in patients who have primary taste and smell complaints. There are many referral centers that specialize in the management of taste and smell disorders (see Chapter 12). There are now commercially available filter paper strips impregnated with sweet, sour, salty, and bitter in different concentrations (taste strips). It is seldom necessary or practical to examine taste on the posterior third of the tongue.
The most common situation calling for assessment of taste is the evaluation of facial nerve palsy. If a patient with a peripheral pattern of facial weakness has impaired taste, the lesion is proximal to the junction with the chorda tympani. A lesion at or distal to the stylomastoid foramen (e.g., in the parotid gland) does not affect taste.
Ageusia is the complete inability to taste. With hypogeusia, taste perception is blunted or delayed. Perversions or abnormal perceptions of taste are parageusias. There is marked individual variation in taste. Complete ageusia is rare unless there is also loss of smell. If there is loss of taste, one should first eliminate the possibility of disease of the tongue. Some causes of disturbed taste are listed in Table 16.3. There are many medications that reportedly alter taste; some commonly used in neurologic practice include the following: carbamazepine, phenytoin, tricyclic antidepressants, dexamethasone, hydrocortisone, penicillamine, lithium, methotrexate, levodopa or levodopa/carbidopa, clozapine, trifluoperazine, baclofen, and dantrolene.
TABLE 16.3 Possible Causes of Disturbed Taste

Modified from Bromley, SM. Smell and taste disorders: a primary care approach. Am Fam Physician 2000;61:427–436, 438.
Examination of the Secretory Functions
The secretory functions of CN VII can usually be evaluated by history and observation. Increased tearing is usually apparent; decreased tearing may be determined from the history. Tear production may be quantitated with the Schirmer test. Commercially available filter strips are placed in the inferior conjunctival sac and left in place for 5 minutes. The advancing edge of moisture down the strip is proportional to the moisture in the eye; the results are expressed in millimeters. This test is simple and does not require referral to an ophthalmologist.
The lacrimal reflex is tearing, usually bilateral, caused by stimulating the cornea. The nasolacrimal reflex is elicited by mechanical stimulation of the nasal mucosa, or by chemical stimulation using irritating substances such as ammonia. Abnormalities of salivation are usually suggested by the history. Otolaryngologists and oral surgeons can use special techniques to quantitate salivary flow.
DISORDERS OF FUNCTION
Motor abnormalities, either weakness or abnormal movements, account for the preponderance of clinical abnormalities of facial nerve function. Changes in sensation, primarily taste, and in secretory function, sometimes occur as a sidebar, but are rarely if ever the major manifestation of disease of CN VII. Changes in these functions can help to localize the lesion along the course of the nerve, although this exercise has little practical value. The major branches in sequence are the greater superficial petrosal, nerve to the stapedius, and chorda tympani, after which the nerve continues to the facial muscles. The mnemonic tear-hear-taste-face may help recall the sequence.
Facial Weakness
There are two types of neurogenic facial nerve weakness: peripheral, or lower motor neuron; and central, or upper motor neuron. PFP may result from a lesion anywhere from the CN VII nucleus in the pons to the terminal branches in the face. Central facial palsy (CFP) is due to a lesion involving the supranuclear pathways before they synapse on the facial nucleus. PFP results from an ipsilateral lesion, whereas CFP, with rare exception, results from a contralateral lesion.
Peripheral Facial Palsy
With PFP, there is flaccid weakness of all the muscles of facial expression on the involved side, both upper and lower face, and the paralysis is usually complete (prosopoplegia). The affected side of the face is smooth; there are no wrinkles on the forehead; the eye is open; the inferior lid sags; the nasolabial fold is flattened; and the angle of the mouth droops (Figure 16.4). The patient cannot raise the eyebrow, wrinkle the forehead, frown, close the eye, laugh, smile, bare the teeth, blow out the cheeks, whistle, pucker, retract the angle of the mouth, or contract the chin muscles or platysma on the involved side. She talks and smiles with one side of the mouth, and the mouth is drawn to the sound side on attempted movement. For videos of PFP see http://www.metacafe.com/watch/1269826/bells_palsy/ and http://www.medclip.com/index.php?page=videos§ion=view&vid_id=101626. The cheek is flaccid and food accumulates between the teeth and the paralyzed cheek; the patient may bite the cheek or lip when chewing. Food, liquids, and saliva may spill from the corner of the mouth. The cheek may puff out on expiration because of buccinator weakness. The facial asymmetry may cause an apparent deviation of the tongue (see Chapter 20 and Figure 15.7).
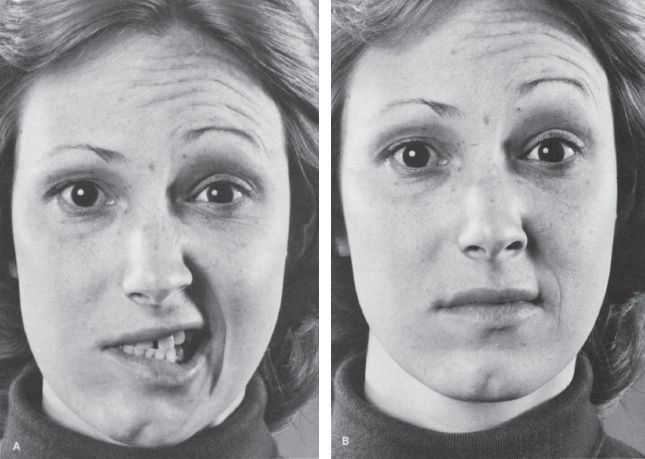
FIGURE 16.4 A patient with a peripheral facial nerve palsy on the right. A. Patient is attempting to retract both angles of the mouth. B. Patient is attempting to elevate both eyebrows.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







