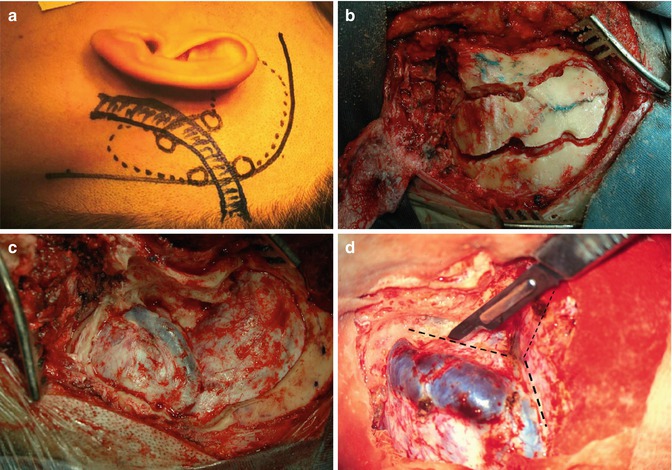
Fig. 19.1
(a, b) Cerebellopontine angle meningioma. (c) Low clivus meningioma. (d–f) Petroclival meningioma
19.3 History
Classically, posterior fossa meningiomas were classified in 1953 by Castellano and Ruggiero [5] using Olivecrona’s series in five groups: cerebellar convexity, tentorium, posterior surface of petrous bone, clivus, and foramen magnum. After the development of computerized tomography, this classification was revised. Yasargil et al. [37] in 1980 suggested that these tumors arise along the petroclival line and classified them into clival, petroclival, sphenopetroclival, foramen magnum, and cerebellopontine angle meningiomas. Mayberg and Symon in 1986 [15] published a series using the terminology clival and petrous apical meningiomas. Since this time many publications used the term petroclival meningioma for those tumors. Cerebellopontine angle (CPA) meningiomas must be excluded from these series because they represent a separate group with different surgical implications. They arise posterior to the sigmoid sinus displacing the cranial nerves VII, VIII, IX, X, and XI anteriorly. Surgical removal of these lesions is usually easier than in cases of petroclival meningiomas.
19.4 Diagnosis
19.4.1 Clinical Symptoms
Petroclival meningiomas are slow-growing tumors that usually produce clinical symptoms after reaching large size. According to Bricolo et al. [4], it takes between 2.5 and 4.5 years from the beginning of symptoms to establish the correct diagnosis. This fact delays diagnosis and treatment. Indication for surgical removal of small tumors is controversial because even small petroclival meningiomas require a major surgical procedure that might increase morbidity, making patients worse after surgery. Clinical symptoms are related to involvement of the cranial nerves, compression of the brain stem and cerebellum, and increase of intracranial pressure. Headache and gait disturbances followed by hearing loss and facial paresthesia are the most frequent symptoms presented by these patients. Symptoms progression is relentless during a long period of time which may delay diagnosis. The trigeminal nerve is more frequently affected followed by IX and X cranial nerves. Facial nerve disturbance will occur in 30 % of patients. Visual disturbances, cerebellar signs, and somatomotor deficits usually occur late due to progression of the disease. Patients with hydrocephalus requiring treatment are rare, because these slow-growing lesions will produce late CSF-pathways occlusion when the tumor has achieved a large size. Characteristically, patients with petroclival meningioma may present good hearing in contrast to severe trigeminal and caudal cranial nerves involvement.
19.4.2 Neuroradiological Evaluation
CT scan examination may reveal bone erosion, hyperostosis, or both. It is useful for evaluation of the bony structures of the skull base. The tumor is usually slightly hyperintense in comparison with the brain tissue and shows strong contrast enhancement. These meningiomas frequently infiltrate the dura mater and present a large implantation (Fig. 19.2). Calcifications may also be observed. MRI is the radiological examination that shows very precisely the extension and relationship of the lesion with the brain stem, vessels, and also cranial nerves (Fig. 19.2). Displacement and compression of normal structures are clearly demonstrated by MRI. In most cases strong gadolinium enhancement is observed. Signs of dura mater infiltration (“dura tail”) may be present. Edema may appear in T2-weighted images showing edema means tumor invasion of the arachnoid has occurred. Tumor vascularity and its relation to major vessels are demonstrated by magnetic resonance angiography (MRA) or computed tomography angiography (CTA). For surgical planning, high-definition MRI studies are of fundamental importance. Four-vessel digital subtraction angiography (DSA) shows the vascular supply, displacement, and involvement (narrowing) of basilar artery and internal carotid artery and their branches. Usually, these tumors are fed by branches of the external carotid artery and the meningohypophyseal trunk (Bernasconi-Cassinari artery) is enlarged. Hypervascular tumors may be preoperatively embolized with particles or glue.
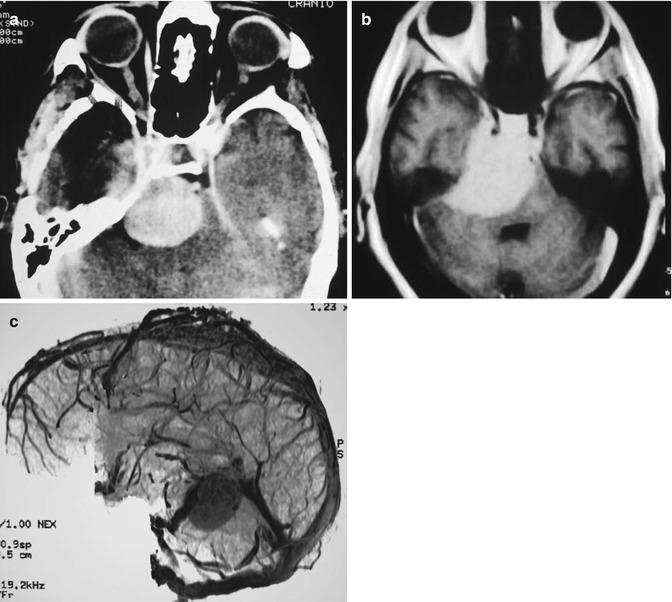

Fig. 19.2
(a) CT scan with contrast showing a large petroclival meningioma; (b) MRI with Gd showing the cavernous sinus infiltration; (c) Venous anatomy on MRA
19.5 Natural History
The natural history of meningiomas is characterized by progressive growth causing compression and involvement of neighboring structures. Van Havenbergh et al. [36] studied 21 patients with petroclival meningiomas treated conservatively. There was a minimum follow-up of 4 years. They found tumor growth in 76 % of cases and clinical impairment in 63 %. Jung et al. [10] reported a series of 38 patients with subtotal removal. They followed these patients and found a linear growth of 0.37 cm/year and a volumetric increase of 4.94 cm3/year. These authors reported, however, that 60 % of patients showed no clinical signs of disease progression. Terasaka et al. [35] managed 15 patients with petroclival meningiomas with a median follow-up period of 40 months (range, 5–170). Sixty percent of the cases showed radiological tumor growth during the follow-up period, and there was functional deterioration in 47 % of the cases.
19.6 Management
Surgical removal of large petroclival meningiomas presents the neurosurgeons an enormous challenge. The operative mortality was very high but could be reduced with development of new diagnostic and surgical procedures [4, 8, 12, 16, 19, 23, 36, 38]. In spite of all recent advances in neurosurgery, neuroradiology, and radiotherapy, the morbidity of surgical treatment of large petroclival meningiomas remains high. Management of petroclival meningiomas includes three options: clinical observation, total removal, and subtotal surgical ablation with or without adjunctive therapy. Clinical observation may be indicated for patients in poor general clinical condition or advanced age. Asymptomatic very small tumors may be followed with MRI every 6 months. Another reason for conservative management is patient’s refusal to surgery. According to our experience, removal of small, minimally symptomatic petroclival meningiomas is indicated because these lesions have the best chance of total resection with no mortality or morbidity. With the strategy of “wait and scan,” in these cases, the tumor can grow, involving cranial nerves and vessels, making tumor dissection from these structures impossible.
Radiotherapy and radiosurgery may be indicated as initial treatment or after partial surgical removal. Complications of radiotherapy for lesions in and around the brain stem have been described. Our strategy is to follow patients with subtotal removal with MRI studies every 6 months. Radiotherapy is recommended only when clear signs of tumor growth are demonstrated.
19.7 Surgical Treatment
Total surgical removal is the only treatment that may cure this benign tumor; however, it may cause disability and mortality. The pros and cons of surgery, radiotherapy, and conservative management are thoroughly discussed with the patients before surgery. The main factor limiting radical removal with preservation of involved structures is invasion of the pia mater. These cases have no clear cleavage plane to permit safe dissection of the cranial nerves and vessels. Small perforating brain stem vessels embedded in the lesion may be damaged during dissection causing brain stem infarction. In these tumors a small piece of tumor around these structures is left behind to avoid postoperative deficits. Cavernous sinus invasion may be a cause of subtotal removal.
19.7.1 Selection of Surgical Approach
The best surgical approach also has to offer the shortest approach with enough exposure and possible detachment of tumor feeders [11]. Choice of surgical approach is usually based on tumor location and extension, involvement of venous structures (i.e., vein of Labbé), and experience of the surgeon. An additional factor to be considered is the shape of the skull. Patients with brachycephalic skull have a shorter anterior-posterior distance to petrous apex, and the middle fossa fronto-orbito-zygomatic approach may be indicated (Fig. 19.3). Dolichocephalic patients are best suited for petrosal approaches because the lateral distance to petrous apex is shorter. Larger tumors invading the cavernous sinus with extension into the posterior fossa may be removed in two surgeries through different approaches. Elderly patients may tolerate better two short-duration surgeries than one long-duration procedure.
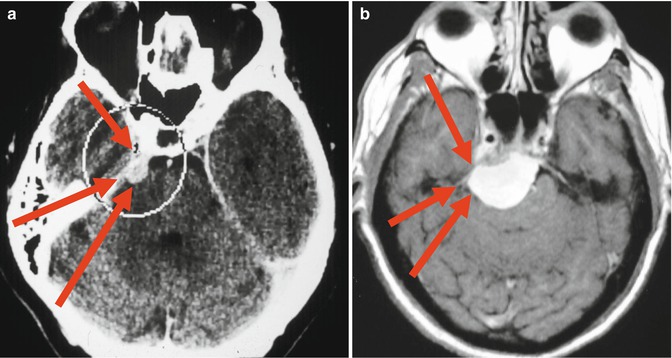

Fig. 19.3
Distances to petrous apex. (a) Brachycephalic skull; (b) “Normal” skull. Red arrows showing fronto-orbito-zygomatic, petrosal and retrosigmoid approaches
Three main surgical accesses are used: fronto-orbito-zygomatic, petrosal, and retrosigmoid approaches. They may be used as a single approach or may be combined.
19.7.1.1 Fronto-Orbito-Zygomatic Approach
This approach is utilized for petroclival tumors with main extension in the middle fossa involving the cavernous sinus (Fig 19.4a, b). Advantages of this access are related to wide exposure of the middle fossa and adequate control of the internal carotid artery (ICA). This approach does not provide, however, good tumor exposure in the infratentorial midline and within the posterior fossa below VII and VIII cranial nerves, even with the opening of tentorium margin.
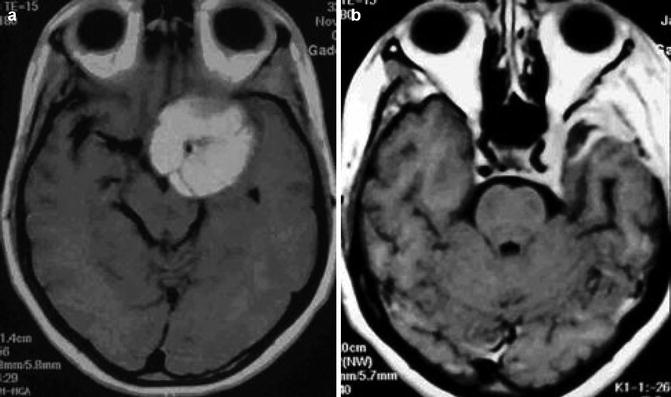

Fig. 19.4
(a) Preoperative MRI showing petroclival meningioma with main extension in the middle fossa. (b) Postoperative MRI after total removal through the fronto-orbito-zygomatic approach
Surgical Technique
The patient is placed in dorsal decubitus with the head turned 30° to the opposite side. Intraoperative monitoring of III, IV, VI, VII, and VIII cranial nerves is performed. A “C”-shaped preauricular skin incision, 1 cm below the zygomatic arch, extending to the frontal area, behind the hairline, is carried out. The skin flap is turned down at the level of temporalis fascia, exposing the root of zygomatic arch, temporomandibular joint, and lateral orbital rim. The ramus frontalis of the facial nerve is preserved. The temporalis muscle is elevated, and a fronto-orbito-temporal craniotomy with the zygomatic arch attached to it is cut in one piece (Fig. 19.5a, b). The temporomandibular joint is opened with preservation of its meniscus. The great sphenoid wing, lateral orbit wall, and base of the middle fossa are removed with a high-speed drill (Fig. 19.5c). The foramen ovale, spinosum, and rotundum are identified. The middle meningeal artery is coagulated and cut. The superior orbital fissure is opened. If necessary, the petrous portion of the ICA is exposed after dissection of the great petrosal nerve, medially to the foramen spinosum and Eustachian tube. Usually we prefer a proximal control of the ICA in the neck. The dura is incised, and the Sylvian fissure is widely opened exposing the ICA and olfactory, optic, and oculomotor nerves (Fig. 19.6a, b). Careful dissection of the carotid bifurcation, anterior and middle cerebral arteries, is the next step. Frequently these structures are involved by tumor. In order to preserve these vessels, dissection begins in the noninvolved portion and dissected from the tumor capsule. The optic canal is drilled out with diamond burs. After identification and dissection of all neurovascular structures, the tumor in the middle fossa is removed exposing the lateral wall of the cavernous sinus. If the cavernous sinus is infiltrated, its lateral wall is opened through a pre- or subtemporal access. A “peeling” of the dura of the lateral wall of the cavernous sinus is performed (Fig. 19.6c). The III cranial nerve and the branches of trigeminal nerve are identified and dissected. The ICA and abducens nerve may be totally involved by tumor within the cavernous sinus (Fig. 19.7a, b). Intraoperative micro-Doppler may be useful to identify the ICA. Intracavernous branches of ICA may be enlarged feeding the tumor. A high-flow bypass between the external carotid artery and the middle cerebral artery (M2 segment), with radial artery or saphenous vein graft, is performed if the ICA will be removed with the lesion (Fig. 19.8a, b). Tumors with anterior extension into the superior orbital fissure may infiltrate the cranial nerves. The objective of surgery is total tumor removal with preservation of ICA and II and III cranial nerves. If the tumor capsule is very adherent to these structures without clear arachnoidal plane or they are infiltrated by the tumor and the nerves are still functioning, a subtotal removal is performed.
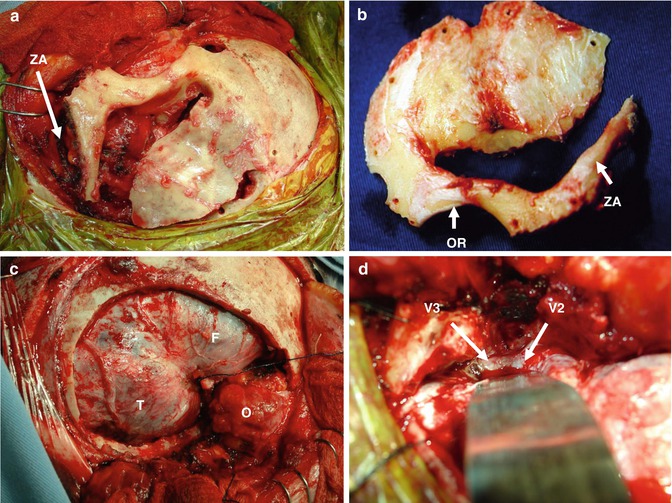
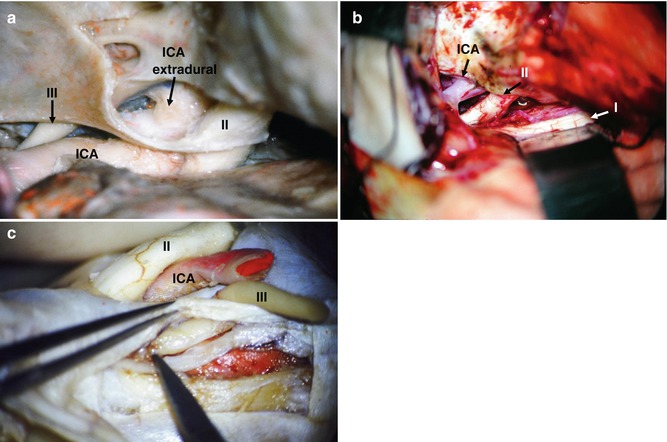
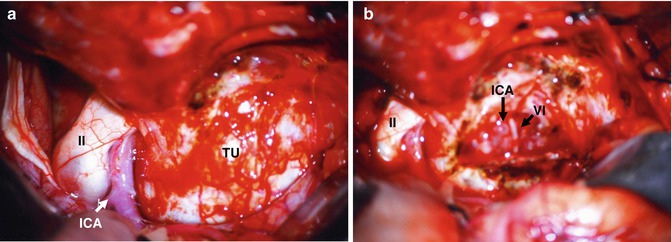
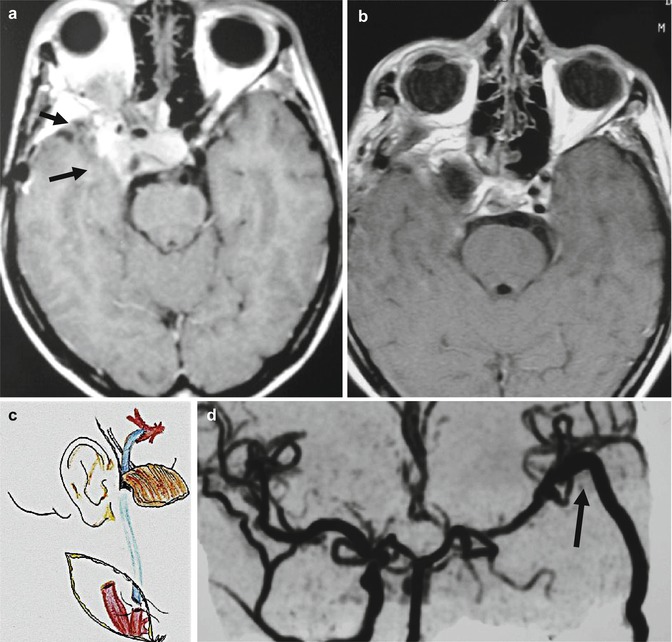

Fig. 19.5
(a) One-piece craniotomy, zygomatic arch (ZA). (b) Craniotomy flap, orbital rim (OR). (c, d) Extradural exposition. Frontal lobe (F), temporal lobe (T), orbit (O). Second and third branches of trigeminal nerve (V2, V3)

Fig. 19.6
(a) Anatomical specimen: extradural ICA exposure, oculomotor nerve (III), optic nerve (II). (b) Surgical picture. (c) Anatomical specimen – “peeling” of CS wall

Fig. 19.7
Intraoperative pictures: (a) Tumor within the cavernous sinus causing bulging of the lateral CS wall (TU), optic nerve (II). (b) After tumor removal, ICA and abducens nerve (VI)

Fig. 19.8
(a) Petroclival meningioma with cavernous sinus invasion. The ICA is embedded by the tumor (arrows). (b) Postoperative MRI after total removal. (c, d) (c) High-flow bypass performed between the ECA and middle cerebral artery with radial artery graft. (d) Postoperative angiography showing ECA and MCA bypass (arrow)
Tumor extension within the posterior fossa is removed after opening the tentorium behind the entrance of the III cranial nerve in the cavernous sinus. The petrous apex and the posterior clinoid process are removed with diamond burs to enlarge exposure. The basilar artery and its branches and the VII and VIII cranial nerves are dissected from the tumor capsule. An adequate surgical exposure is not possible below the level of the internal auditory canal. Tumor exposure in the mid- and lower portions of the clivus is not adequate with this approach. After removal of the lesion, the dura mater is closed in watertight fashion, and the craniotomy flap is replaced and fixed to the skull. The wound is closed in routine fashion.
19.7.1.2 Petrosal Approach
Indication
The petrosal approaches are presigmoid retrolabyrinthine, presigmoid translabyrinthine, and total petrosectomy. These approaches are used when the lesion is located in the posterior, middle fossa, and clivus region (Fig. 19.9a–d). If the patient has preoperative good hearing, we prefer the presigmoid retrolabyrinthine access. When there is no hearing, it is possible to remove the semicircular canals and this approach is called presigmoid translabyrinthine. For giant tumors crossing the midline in the prepontine region, a more lateral and extensive approach is necessary. These cases are operated through total petrosectomy.
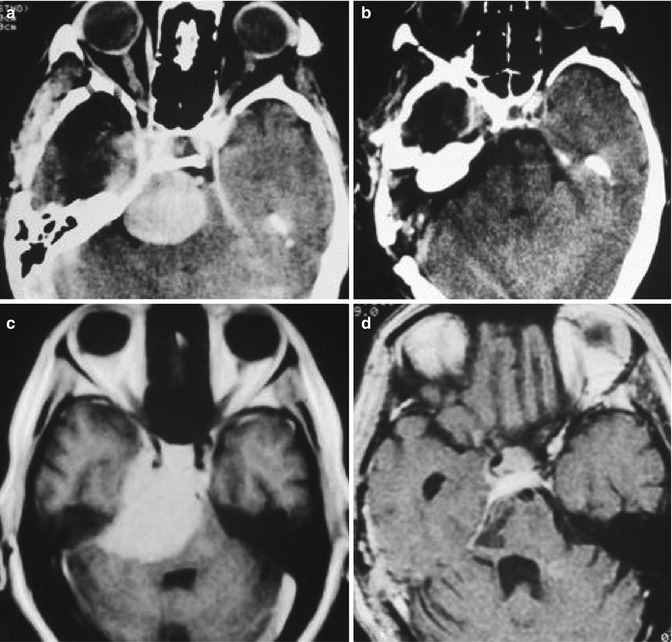

Fig. 19.9
Preoperative (a, c) and postoperative (b, d) MRI of petroclival meningiomas removed through presigmoid approach
Surgical Technique
Presigmoid (Retrolabyrinthine) Approach
Surgery is performed with the patient in dorsal decubitus with the head rotated to the opposite side. A semicircular skin incision from the temporal region, 4 cm above the zygomatic arch, passing 3 cm behind the ear, extending 2 cm behind the mastoid tip is performed (Fig. 19.10a). To avoid postoperative CSF leak, a skull base reconstruction technique utilizing fascia/muscle flaps was developed. The temporalis muscle fascia is cut and dissected with the mastoid periosteum, the cranio-cervical fascia, and the sternocleidomastoid muscle that is detached from its insertion. These structures form a large vascularized muscle/fascia flap that is rotated back at the end of surgery to cover the entire surgical field. The temporal muscle is dissected in the anterior direction and used to cover the dura mater, as a vascularized muscle, at the end of the operation. The cortical bone of the mastoid is drilled out exposing the antrum, tegmen mastoid, and sinus plate. The labyrinth block and the facial nerve canal are identified. These canals are not opened. Two trepanations above and two below the sigmoid sinus are placed, and with a high-speed drill, a craniotomy exposing the middle fossa and the posterior fossa (retrosigmoid) is cut (Fig. 19.10b). The superior petrosal, the sigmoid, and the transverse sinuses are exposed. The retrofacial mastoid cells down to the jugular bulb are removed. The dura mater anterior to the sigmoid sinus is exposed (Fig. 19.10c). The zygomatic and supra-labyrinthine cells are removed maintaining the semicircular canals and middle ear intact. The superior petrosal sinus is coagulated and ligated between two stitches. The dura mater is incised anterior to the sigmoid sinus and parallel to the middle fossa floor (Fig. 19.10d). The superior petrosal sinus is cut. The tentorium is severed initially perpendicular to the superior petrosal sinus in a length of 2 or 3 cm and then medially, parallel to the transverse sinus for an additional 3 cm. This maneuver allows a wide exposure of the cerebellum separating it from the posterior aspect of temporal lobe like “opening a book” (Fig. 19.11a). Care should be taken to preserve the vein of Labbé which has a variable anatomy and usually enters the transverse sinus 10 mm before its junction with the sigmoid sinus. Preoperative evaluation of the venous anatomy is necessary in planning this approach. The tentorium incision is continued up to the incisura where the IV nerve is exposed and preserved (Fig. 19.11b). Some small basal temporal lobe bridge veins are coagulated and cut allowing wide subtemporal exposure. Two brain spatulas are placed supporting the temporal lobe and the cerebellum exposing the entire petroclival region from the III nerve up to VII and VIII nerves (Fig. 19.11c). Usually the trigeminal nerve can be seen displaced posterior and superiorly. The tumor is devascularized by bipolar coagulation of its dural insertion. Intracapsular, piecemeal tumor resection using the ultrasonic aspirator is the next surgical step. Tumor debulking allows dissection of tumor capsule from the nerves and basilar, superior cerebellar, and posterior cerebral arteries. The abducens nerve is very thin and fragile. Dorello’s canal is located medially to the VII and VIII cranial nerves. This region should be approached only after wide resection of the tumor. Tumor extension into the posterior portion of the cavernous sinus is resected following the trigeminal nerve. All infiltrated petrous and clivus bone are removed with a diamond drill. After total removal (Fig. 19.11d, e), the dura mater is closed in watertight fashion or with fascia graft and fibrin glue. The aditus and antrum are closed with a muscle plug, and the temporalis muscle is flap rotated to cover the entire exposed dura mater. The fascia-muscle flap (temporalis, cranio-cervical fasciae, and sternocleidomastoid muscle) is sutured in its original position, and the skin is closed.