Phenobarbital and Primidone
Blaise F. D. Bourgeois
HISTORICAL BACKGROUND
Although its use has been decreasing, phenobarbital is still a major antiepileptic drug (AED). Phenobarbital (PB) has been prescribed for the treatment of epilepsy since 1912, with only bromide having been used longer. Although PB is associated with more sedative and behavioral side effects than most other AEDs, it has relatively low systemic toxicity and a long half-life, can be administered intravenously and intramuscularly, is effective in patients with status epilepticus, and is inexpensive.
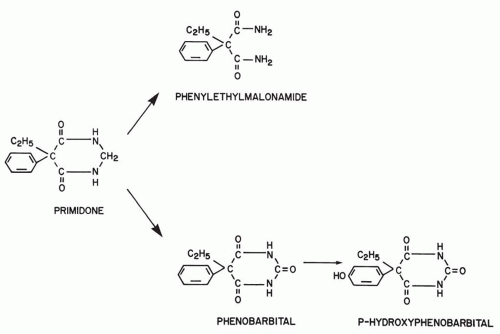
Primidone (PRM) has been in clinical use since its synthesis in 1952 (1). Often referred to as a barbiturate, PRM does not strictly belong in this class; its pyrimidine ring contains only two carbonyl groups, compared with the three groups of barbituric acid (Fig. 56.1), but the remainder of the structure is identical to that of PB. Therapeutically, however, PRM is appropriately considered a barbiturate, as its effect can be attributed predominantly to the derived PB. This hepatic biotransformation has heretofore made it impossible to establish whether therapy with PRM differs clinically from that with PB or whether PRM is a PB prodrug. Complicating this issue is the experimental demonstration of independent antiepileptic activity for the other main metabolite of PRM, phenylethylmalonamide (PEMA) (Fig. 56.1).
CHEMISTRY AND MECHANISM OF ACTION
Chemically, PB is 5-ethyl-5-phenylbarbituric acid (Fig. 56.1). The molecular weight is 232.23, and the conversion factor from milligrams to micromoles is 4.31 (1 mg/L = 4.31 μmol/L). The sodium salt of PB is water soluble. PB in its free acid form is a white crystalline powder soluble in organic solvents, but with limited water and lipid solubility; it is a weak acid with a pKa of 7.3. Many actions of PB at the cellular level have been described. Although it is not certain which are responsible for seizure protection, the available evidence seems to favor enhancement of γ-aminobutyric acid (GABA) inhibition (2). In animal models, PB protects against electroshock-induced seizures and, unlike phenytoin, carbamazepine, and PRM, against seizures induced by such chemical convulsants as pentylenetetrazol. In normal animals, PB raises the threshold and shortens the duration of afterdischarges elicited by electrical stimulation (3). Like other barbiturates, PB enhances postsynaptic GABAA receptor-mediated chloride (Cl−) currents by prolonging the opening of the Cl− ionophore (4). Increased flow of Cl− into the cell decreases excitability. Presynaptically, PB can cause a concentration-dependent reduction of calcium (Ca2+)-dependent action potentials (5). Usually occurring at relatively high concentrations, this may contribute to seizure protection at higher therapeutic levels and, especially, to sedative and anesthetic effects.
Chemically, PRM is 5-ethyldihydro-5-phenyl-4,6(1-H, 5H) pyrimidinedione. The molecular weight is 218.264, and the conversion factor from milligrams to micromoles is 4.59 (1 mg/L = 4.59 μmol/L). PRM is very poorly soluble in water, somewhat soluble in ethanol, and virtually insoluble in organic solvents.
The basic pharmacologic mechanism of action of PRM has received relatively little attention, not least because it was uncertain for some time whether the agent itself has independent antiepileptic activity. The basic anticonvulsant action of PRM has been studied in mouse neurons in cell culture (6). PRM was compared with PB for its effect on amino acid responses and on sustained, high-frequency
firing. In contrast to PB, PRM had no effect on postsynaptic GABA and glutamate responses at concentrations up to 50 μg/mL. However, both agents limited sustained, high-frequency, repetitive firing at relatively high concentrations (>50 μg/mL). Together, PRM and PB limited sustained high-frequency, repetitive firing at clinically relevant concentrations (12 μg/mL and 20 μg/mL, respectively). The authors concluded that PRM and PB may act synergistically to reduce sustained, high-frequency, repetitive firing. These in vitro findings are in accordance with observations made in whole animals.
firing. In contrast to PB, PRM had no effect on postsynaptic GABA and glutamate responses at concentrations up to 50 μg/mL. However, both agents limited sustained, high-frequency, repetitive firing at relatively high concentrations (>50 μg/mL). Together, PRM and PB limited sustained high-frequency, repetitive firing at clinically relevant concentrations (12 μg/mL and 20 μg/mL, respectively). The authors concluded that PRM and PB may act synergistically to reduce sustained, high-frequency, repetitive firing. These in vitro findings are in accordance with observations made in whole animals.
All the evidence regarding the individual antiepileptic properties of PRM, PB, and phenylethylmalonamide (PEMA) is derived from experiments in animals whose seizures were provoked, because PRM is never present alone during long-term therapy and because at least one active metabolite, PB, is present after repeated administration in humans, as well as in experimental animals. Because the metabolites accumulate a few hours after administration of the first dose, a possible long-term protection by PRM alone against spontaneously occurring seizures cannot be assessed in humans. In addition, PEMA may be involved in the overall pharmacodynamic effect of PRM. The first evidence of the independent anticonvulsant activity of PRM came from dogs who were protected against experimental seizures at a lower concentration of PB when PRM was also present than when PB alone was present (7). Rats were similarly protected against induced seizures after a single dose of PRM administered before the active metabolites could be detected (8), as were mice pretreated with a metabolic blocker that delayed the biotransformation of PRM (9,10). The anticonvulsant potency of PRM against maximal electroshock-induced seizures is similar to that of PB, but unlike PB, PRM was ineffective against chemically induced seizures caused by pentylenetetrazol or bicuculline (9). Thus, the experimental anticonvulsant spectrum of PRM differs from that of PB and is similar to that of carbamazepine and phenytoin; therefore, PRM and PB may be two different AEDs with different mechanisms of action.
On the basis of brain concentrations in mice, PRM appears to be 2.5 times less neurotoxic than PB, with a superior therapeutic index (9). When PB and PRM were administered together in single-dose experiments in mice (11), their anticonvulsant activity was supra-additive (potentiated) and their neurotoxic effect was infra-additive. A PB-PRM brain concentration ratio of 1:1 provided the best therapeutic index. This ratio is not usually seen in patients, especially those taking PRM combined with phenytoin or carbamazepine. If PRM is different from or even better than PB for the treatment of epilepsy, its effect would be likely only when the PRM concentration equals or exceeds the PB concentration. Such a ratio is achieved only rarely with PRM monotherapy and almost never when PRM is added to phenytoin or carbamazepine, or combined with PB. The results of pharmacodynamic interactions between PRM and PB in mice were confirmed by experiments in amygdala-kindled rats. After single doses, the anticonvulsant effect of PB was potentiated by PRM, whereas side effects of PB, such as ataxia and muscle relaxation, were not increased by combined treatment with PRM (12).
In rats (13) and mice (9,10), PEMA had relatively weak anticonvulsant activity of its own. On the basis of brain concentrations in mice (9), PEMA was 16 times less potent than PB in seizure protection and 8 times less potent in neurotoxic effects, but it potentiated the anticonvulsant (11,13) and neurotoxic effects (11) of PB. Nevertheless, a
quantitative analysis of these experimental results, together with the blood levels encountered in clinical practice, suggests that PEMA does not significantly add to the antiepileptic effect or neurotoxicity of PRM therapy.
quantitative analysis of these experimental results, together with the blood levels encountered in clinical practice, suggests that PEMA does not significantly add to the antiepileptic effect or neurotoxicity of PRM therapy.
ABSORPTION, DISTRIBUTION, AND METABOLISM
Phenobarbital
The main pharmacokinetic parameters of PB and PRM are summarized in Chapter 45, Table 45.2. Most formulations of PB contain sodium salt because of good aqueous solubility. The absolute bioavailability of oral preparations of PB is usually greater than 90% (14). Absorption of PB following intramuscular (IM) administration was found to be as complete as that following administration of oral tablets, compared with intravenous (IV) administration (15). Accumulation half-life for the IM route (0.73 hours) was not shorter than for the oral route (0.64 hours). Time to peak concentration is usually 2 to 4 hours. In newborns, however, peak PB plasma levels after oral administration may be reached later than after IM administration (16). A parenteral solution of PB administered rectally has a bioavailability of 89%, compared with that of IM administration (17); average time to peak concentration was 4.4 hours.
PB is not highly bound to serum proteins (45%). Protein binding of PB is lower during pregnancy and in newborns, with a bound fraction between 30% and 40% in pregnant women and their offspring (18). Reported values for the volume of distribution vary. Following IV administration, average values were 0.54 L/kg in adult volunteers and 0.61 L/kg in adult patients with epilepsy (15), both well within the reported range. The volume of distribution of PB approached 1.0 L/kg in newborns (19).
PB is eliminated mostly via renal excretion of the unchanged drug, and via hepatic metabolism and renal excretion of the metabolites. An average of 20% to 25% of PB is eliminated unchanged by the kidneys in adults, with large interindividual variability (20,21). The main metabolite of PB is p-hydroxyphenobarbital (Fig. 56.1). At steady state, approximately 20% to 30% of the PB dose is transformed into this metabolite, approximately 50% of which is conjugated to glucuronic acid (20,21). Nitrogen glucosidation, another relevant pathway of PB metabolism, accounts for 25% to 30% of total PB disposition (22). Other identified metabolites of PB represent a very low percentage of the total elimination.
The elimination of PB from serum follows first-order, or linear, kinetics. PB has the longest elimination half-life of the commonly used AEDs. The half-life of PB is age-dependent. The half-life is usually well above 100 hours in newborns (23) and averages 148 hours in asphyxiated newborns (24). During the neonatal period, PB elimination accelerates markedly; thereafter, half-lives are very short, with average values of 63 hours during the first year of life and 69 hours between the ages of 1 and 5 years (25). Half-lives in adults range between 80 and 100 hours, and no evidence of autoinduction of PB metabolism has been demonstrated (15).
Primidone
PRM is supplied as 250-mg and 50-mg tablets and as syrup (1 mL = 50 mg); extremely low solubility precludes parenteral administration. After oral ingestion of tablets, the time to peak serum concentrations in adult patients with epilepsy was 2.7 (26) and 3.2 hours (27), respectively, and 4 to 6 hours after single-dose administration in children (28). In the same study, an average of 92% of the dose (range, 72% to 123%) was excreted in the urine as unchanged PRM and metabolites, probably indicating complete oral bioavailability. Concomitant administration of acetazolamide reduced the oral absorption of PRM (29). One generic preparation was found to have a lower bioavailability than the trademark product (30).
The volume of distribution of PRM ranged from 0.54 L/kg following acute intoxication (31) to 0.86 L/kg (32). The volume of distribution of PEMA after its oral administration was 0.69 L/kg (33). In human plasma, protein binding of both PRM and PEMA was less than 10% (13,27,33). Brain concentrations of PRM were found to be lower than simultaneous plasma concentrations in mice (9,10) and in rats (8). In patients undergoing surgery for intractable epilepsy, one group of investigators found an average brain-to-plasma ratio of 87% (34). In another report (10) of six patients whose mean plasma PRM concentration was 6.3 μg/mL, brain concentrations ranged between nondetectable and 2.2 μg/g. Brain concentrations of PEMA in mice were 93% (10) and 77% (9) of the plasma levels. In humans, the cerebrospinal fluid-plasma ratio for PRM ranged from 0.8 to 1.13 (27,34,35), which is similar to human saliva-to-plasma ratios (36) and which is consistent with the high free fraction of plasma PRM.
The elimination half-life of PRM varies, mainly because of enzymatic induction by comedication. In adults receiving long-term PRM monotherapy, the elimination half-life ranged from 10 to 15 hours (37, 38, 39). Therapy with additional AEDs was associated with values of 6.5 and 8.3 hours (26,27,38,39). In 12 children (four treated with PRM monotherapy, eight treated with PRM and phenytoin), half-lives ranged from 4.5 to 11 hours (mean, 8.7 hours) (28). In newborns, however, the average PRM half-life was 23 hours (range, 8 to 80 hours) (40), which were associated with a limited biotransformation to the metabolites (41).
After oral ingestion of PEMA itself, the half-life of PRM was 15.7 hours (33). The elimination rate of PEMA cannot be determined accurately in patients taking PRM, because the liver produces PEMA as long as PRM is measurable in the blood.
Because two metabolites of PRM accumulate after repeated administration of the agent and because both have independent anticonvulsant activity, an understanding of the qualitative and quantitative aspects of PRM metabolism is needed before any rational clinical use of this drug can be undertaken. Ideally, before prescribing the agent, the physician should know the relative antiepileptic potency, relative toxicity, and expected relative blood levels of PRM and its two active metabolites. Unfortunately, this information is only partially available. Although relative efficacy and relative toxicity of PRM and its metabolites have been studied acutely in animals (9,11), similar investigations are virtually impossible in humans because the three compounds are always present simultaneously during long-term therapy.
Figure 56.1 shows the relevant metabolic pathways for PRM. The first metabolite of PRM to be identified, PEMA was found initially in rats (42) and thereafter in every species studied. PB and p-hydroxyphenobarbital were discovered only 4 years later, in 1956 (43), and toxic reactions attributed to the derived PB were first reported in 1958 (44). Other metabolites of PRM, with either negligible or nondetectable blood levels during long-term therapy, have shown no practical significance. Numerous clinical studies have discussed the quantitative aspects of the biotransformation of PRM to PB and PEMA. A comparison of the ratios of PB serum levels to dose during long-term PB therapy and during long-term PRM therapy in the same patients demonstrated that 24.5% of the PRM dose is converted to PB (45). This is in accordance with the report that average PRM doses (in milligrams per kilogram per day) required to maintain a given PB level are about 5 times higher than the equivalent PB doses (46). The extent of PRM biotransformation and the ratios of the blood levels of PRM and its metabolites are very sensitive to interactions with other AEDs and are discussed separately.
INTERACTIONS WITH OTHER AGENTS
Most of the interactions of PB reflect its status as an enzymatic inducer that accelerates the biotransformation of some AEDs, as well as other agents. No clinically significant interaction with PB has been reported that involves absorption. Moreover, because PB is only 55% protein bound in serum, significant interactions involving displacement from serum proteins do not occur. Clinically, the most significant interaction affecting PB levels is the inhibition of PB elimination by valproate (47). Seen in the majority of patients, the extent of this interaction is variable, although the increase in PB concentration can reach 100%, often necessitating dosage adjustments. The concentrations of PB derived from PRM are equally affected by valproate.
In the great majority of interactions, PB affects levels of other agents. Levels of valproate (48) and carbamazepine (49) are often reduced by the addition of PB. Levels of the active metabolite of carbamazepine, the 10,11-epoxide, are less affected or may even increase, and the epoxide-carbamazepine ratio is usually higher in the presence of PB. Relative to the metabolism of phenytoin, PB appears to cause both enzymatic induction and competitive inhibition. The two effects tend to balance out in patients, and dosage adjustments of phenytoin are seldom necessary (50). PB significantly increases the clearance of lamotrigine (51), as well as that of ethosuximide, felbamate, topiramate, zonisamide, and tiagabine (52).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree