Photosensitivity: Genetics and Clinical Significance
B. A. Neubauer*
St. Waltz†
M. Grothe†
A. Hahn*
I. Tuxhorn‡
T. Sander§
G. Kurlemann||
U. Stephani†
*Department of Neuropediatrics, Zentrum Kinderheilkunde und Jugendmedizin, Giessen, Germany
†Department of Neuropediatrics, Klinik für Neuropädiatrie der Universität, Kiel, Germany
‡Epilepsy Center Bethel, Klinik Mara, Bielefeld, Germany
§Gene Mapping Center (GMC), Max-Delbrück-Centrum, Berlin, Germany
||Department of Neuropediatrics, University of Münster, Münster, Germany
Introduction
In some of the generalized idiopathic epilepsy (IGE) syndromes, especially those with the strongest genetic background, photosensitivity is found at a significantly increased rate compared to the normal population and to cohorts of patients with focal or symptomatic epilepsy. Photic stimulation may elicit a cortical response ranging from occipital spikes only to generalized spike-waves discharges (type 1 to 4) during EEG recording. In the past, most authors have only recognized generalized discharges as “true” photosensitivity, for its close association with epilepsy. However, family studies demonstrate a common genetic basis for all four types of photosensitivity and favor an autosomal dominant mode of inheritance. In patients with photosensitive epilepsy, cortical control of contrast gain has been found severely impaired. A dopaminergic mechanism was demonstrated neuropharmacologically. The high prevalence of photosensitivity in the normal population, its occurrence in neurodegenerative disorders, and its detection in individuals suffering from idiopathic epilepsies with confirmed defects of different ion-channel genes makes genetic heterogeneity most likely. In a sample consisting of 37 nuclear pedigrees, we conducted a linkage candidate study investigating the loci of EPM1, EPM2, and DRD1 to DRD5 and formerly reported loci of idiopathic epilepsies on 1p, 2q36, 3p14, 3q26, 4p, 6p11, 6p21.3, 8p11, and 15q14. Finally, none of the investigated loci surpassed the limit of proved or suggestive evidence for linkage.
Definition of Photosensitivity
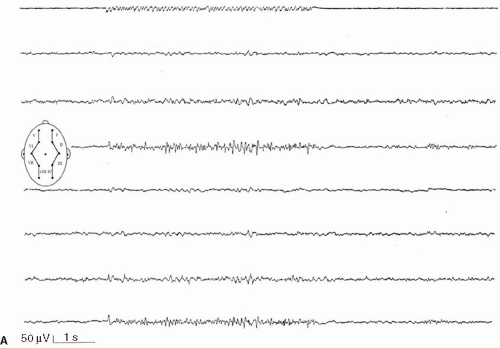
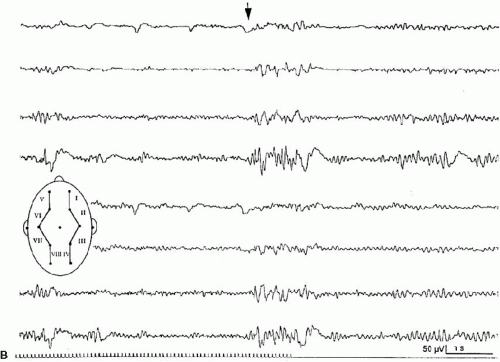
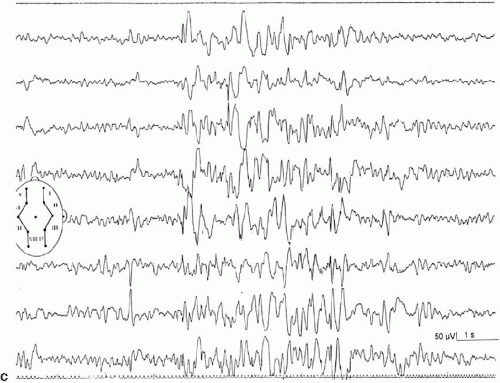
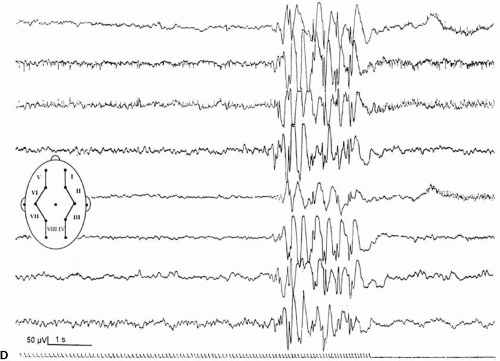
Intermittent photic stimulation (IPS) may elicit different types of cortical responses. Photic driving or photic following, a phase-locked wave form frequently observed at stimulation frequencies ranging from 8 to 10 Hz, has no pathologic implication. Apart from this, several types of cortical responses have been labeled photoparoxysmal response or photosensitivity by different authors. The modalities of stimulation are critical for diagnostic sensitivity. This topic is covered by ample literature (1). An early description of flashlight-induced seizure stimulation dates back to Walther and Walther (2), who found the optimal stimulation frequency to range from 12 to 18 Hz. Bickford et al. (3) finally defined generalized spike-wave activity as the “Photoconvulsive Response,” which is the most frequent description cited. This type of photoparoxysmal response has a close association to epilepsy, especially when it is self-sustaining beyond the period of stimulation (4). However it is known, that this is only the most drastic type of response: Doose and colleagues graded photosensitivity into four types [Doose et al. (5,6), Waltz et al. (7), Doose and Waltz (8)]. Type 1 is characterized by purely occipital spikes within the occipital alpha rhythm. In type 2, parietooccipital spikes are followed by a biphasic slow wave. Type 3 consists of parietooccipital spikes followed by a biphasic slow wave spreading to the frontal region. In type 4, which is synonymous to the photoconvulsive response of Bickford, generalized spike-wave and polyspikes occur (Fig. 16-1). This classification was not based on formal definition, but on different types of photoparoxysmal responses found in a multiplex of families of patients with epilepsy. The trait is age dependent with a maximum penetrance and expression between the ages of 9 to 16 years and is more frequent in girls than in boys (9). It may be mentioned that the so-called photomyoclonic or photomyogenic response is not related to the photoparoxysmal response. It consists of strictly stimulus synchronous cloni of the periorbital muscle groups, while the EEG, besides the muscle artifacts, remains unremarkable (10).
Photosensitivity and Epilepsy
Starting from the historic definition of photosensitivity, it is now obvious that only the type 4 response is strongly associated with seizures. Binnie (1) argues with Reilly and Peters (4) that in the setting of an EEG laboratory of an epilepsy unit, the a priori probability for epilepsy might be 50 times higher than in the normal population. Under these circumstances, a type-4 photosensitivity has a high prognostic value concerning the risk of seizures, possibly reaching up to 80%. Precise numbers given by different authors are difficult to compare for a variety of technical and ascertainment biases. If the setting of an EEG laboratory in an epilepsy unit is left and only healthy individuals are investigated, the epilepsy risk may possibly be much lower, even in type-4 photosensitivity. If all four types of photosensitivity are considered jointly and nonepileptic children are included (family studies), the relative risk might not be higher than 3% (8).
Categorically, epilepsy in individuals with photosensitivity can be divided into pure photosensitive epilepsy, epilepsy with photosensitivity, and photosensitivity in the EEG only, not accompanied by any documented seizures. It should be noted that only 50% of probands with a type-4 photosensitivity, who experience a video documented, mostly self-limiting seizure, will have a recollection of this (11,12); these reports include generalized tonic–clonic seizures in 84%, absences in 6%, and partial motor seizures in 1.5%. Television or video-game epilepsy is the most prominent example of seizures induced by optic stimulation (13). Photosensitivity is found as a frequent feature in several epilepsy syndromes like JME, eyelid myoclonia with absences, severe myoclonic epilepsy (Dravet syndrome), and myoclonic-astatic epilepsy (Doose syndrome). Some authors argue that, depending on the mode and intensity of photic stimulation in patients with juvenile myoclonic epilepsy (JME), the photoparoxysmal response may be provoked in up to 90% of patients (14), while 30% is the order of magnitude documented under the standard stimulation procedure (15). It is also known that a number of progressive myoclonus epilepsies are almost invariably associated with photosensitivity (2). In Lafora disease and Unverricht–Lundborg syndrome, the photoparoxysmal response closely resembles the one observed in idiopathic epilepsies.
Pathomechanism
The cortical reaction to photic stimuli is most likely controlled by a complex network, which involves several mechanisms and components. The precise pathophysiology of photogenic-induced EEG discharges or seizures is far from clear. However, some basic aspects have been elucidated. It is known that stroboscope flashes with a frequency ranging from about 10 to 20 Hz are most provocative and that a distinct proportion of probands with confirmed photosensitivity also shows pattern sensitivity (16). This may serve as a model that is apparently more suited for neurophysiologic investigation of photosensitivity than pure flash stimulation by itself. It has been shown by Wilkins et al. (17) [concisely reviewed by Binnie (1)] that at least 40% black–white contrast with lined contours at a special frequency of 2 to 4 cycles per degree are most epileptogenic. Binocular fusion, steady neuronal synchronization (pattern may not drift through the visual field), and oscillation support the generation of occipital discharges.
In a series of experiments in 11 patients with photosensitive epilepsy (PSE), Porciatti and co-workers (18) showed that increased contrast of the presented stimulus resulted in an almost constant increase of steady-state VEP amplitude. In normal controls, this response leveled out into a plateau at a much lower contrast of the stimulus and could not be increased to the level observed in the probands. The authors concluded that the cortical mechanism for controlling responses to contrast gain at low temporal frequency and high luminance is severely impaired in patients with PSE. It has also been shown, that apomorphine (a dopaminergic drug) abolishes photosensitivity transiently (19,20). There has been a debate concerning the question whether occipital spikes only (i.e., type 1) and generalized discharges (i.e., type 4), both elicited by visual stimuli, represent different grades of expression of one common mechanism, or are two discrete entities. Several authors have addressed this question and have shown that different modes of stimulation affect the propagation of both responses in a diverging manner (21). However, in our opinion, the conclusion that both responses result from entirely separate mechanisms is too far reaching. Indeed, results obtained by family studies clearly demonstrate common genetic components in both types of responses (7).
Genetics
The photoparoxysmal response (PPR) is genetically determined. Over the last decades, case studies of more than 10 independent pairs of monozygotic twins concordant for photosensitivity were reported [e.g., Herrlin (22)]. Based on the fact that these twin pairs were not ascertained systematically and because negative concordant twin pairs will most likely fail to be reported, the degree of heritability cannot be deduced from these reports. Different stimulation techniques and diverging trait definitions render data of family studies difficult to compare. Doose and Gerken (23) studied the EEG of 645 siblings of mostly epileptic patients to determine the heritability of different EEG traits. The siblings of photosensitive probands with and without epilepsy showed a high rate of cortical response to photic stimulation. In consecutive studies Waltz et al. (7) and Doose and Waltz (8) graded the photoparoxsymal response into four types on the basis of family studies (Fig. 16-1). It was demonstrated that the incidence of PPR in siblings of probands with PPR type 4 was identical to the incidence found in siblings of probands with types 1–3. Furthermore, the quantitative expression of photosensitivity turned out to be age dependent. Relatives up to the age of 10 years displayed significantly more often type 3 and type 4 discharges than siblings older than 15 years did. Taken together, these data clearly show that the four different types of PPR represent different levels of expression of the identical genetic trait (8).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree