Figure 5.1. Mesial temporal sclerosis. Coronal T2-weighted image from MRI without gadolinium in a 7-year-old male with temporal lobe epilepsy shows increased signal intensity and decreased size of the left hippocampal formation (arrow).
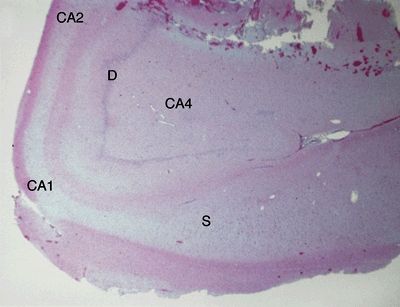
Figure 5.2. Low-magnification appearance of hippocampus in hippocampal sclerosis (HS). An adult patient who underwent anterior temporal lobectomy for treatment of intractable temporal lobe epilepsy. HS is the most common cause of intractable partial epilepsy in adults. HS is generally marked by preferential loss of neurons in the dentate (D), CA4 region, CA1 region, and subiculum (S). A lesser degree of neuronal loss may be observed in the CA3 and CA2 regions. Loss of neurons is accompanied by gliosis and, in severe cases, grossly evident atrophy.
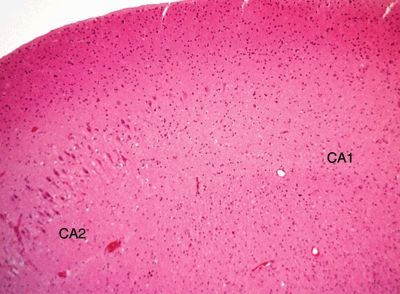
Figure 5.3. Higher-magnification appearance of the hippocampus in hippocampal sclerosis at the interface between CA2 and CA1 regions. There is a marked loss of neurons in the CA1 region with gliosis.
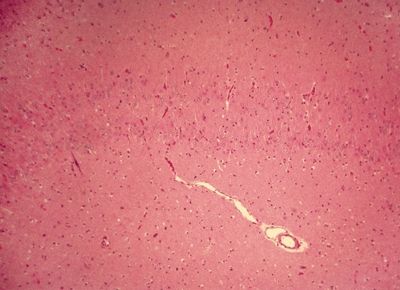
Figure 5.4. Histologic appearance of double dentate marked by two bands of neurons in the hippocampus. This represents a form a hippocampal dysplasia. Hippocampal dysplasia is an infrequent cause of temporal lobe epilepsy and may be seen as a dysmorphic hippocampal formation on a high-definition three-dimensional volume acquisition sequences on brain MRI.
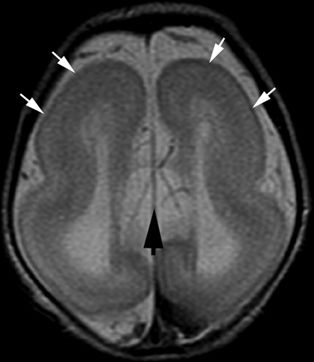
Figure 5.5. Lissencephaly. Axial T2-weighted image from MRI without gadolinium in a newborn shows lack of normal sulcation (white arrows), parallel lateral ventricles, and absence of the corpus callosum (black arrow). Children with lissencephaly usually present with epileptic spasms, severe global developmental delay, microcephaly, and marked hypotonia during early infancy.
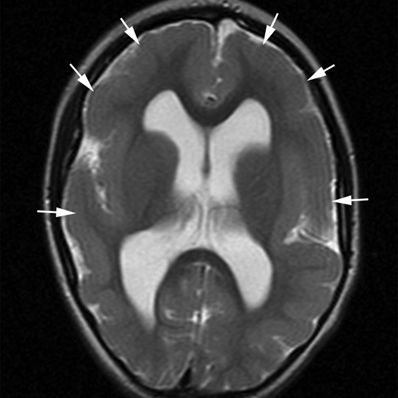
Figure 5.6. Pachygyria. Axial T2-weighted image from MRI without gadolinium in a 4-year-old with spastic quadriplegia and generalized seizures shows a paucity of sulcal markings and thickened cortex bilaterally (arrows).
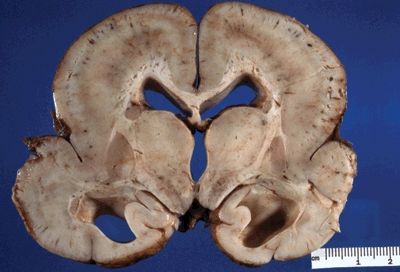
Figure 5.7. The gross appearance of lissencephaly (agyria) characterized by a lack of gyral formation and a decreased number of sulci. Note enlargement of ventricles, suggesting parenchymal volume loss. The cortex is usually thickened on cross-section. Microscopically, there is an abnormally layered cortex, typically three to five layers.
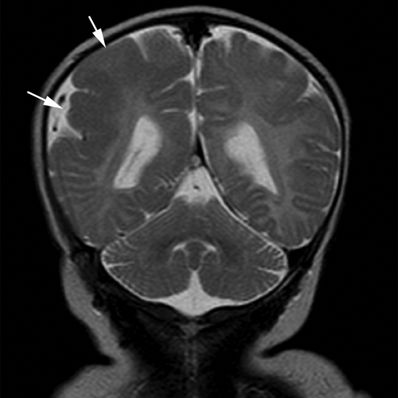
Figure 5.8. Polymicrogyria. Coronal T2-weighted image from MRI without gadolinium in a newborn with motor seizures shows generalized thickening of the cortex of the right parietal lobe characterized by multiple small gyri (arrows). Polymicrogyria are usually epileptogenic lesions, sporadic or familial in occurrence, and various brain MRI patterns have been recognized that help in making an accurate diagnosis.
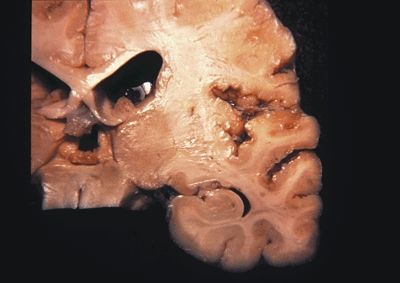
Figure 5.9. Gross appearance of perisylvian polymicrogyria (micropolygyria) marked by the focal presence of small, irregular gyri separated by shallow sulci. The cortex is often thinned and microscopically is composed of two- to four-layered cortex. The leptomeninges overlying polymicrogyria may be abnormally hypervascular due to a persistence of fetal leptomeningeal vascularization. Congenital bilateral perisylvian polymicrogyria (CBPP) usually presents with seizures during childhood. Other clinical findings in the patients with CBPP include pseudobulbar paresis, dysarthria, swallowing difficulties, and tongue paresis with inability to protrude tongue and to perform lateral tongue movements. (Photograph courtesy of Dr. Bette Kleinschmidt-DeMasters.)
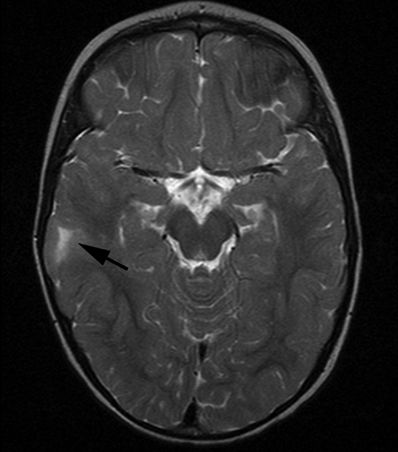
Figure 5.10. Balloon cell dysplasia. Axial T2-weighted image from MRI without gadolinium in an 18-month-old male with intractable seizures shows high signal in the right parietal subcortical white matter subtending a broad-based gyrus (arrow). Most focal cortical dysplasias are sporadic congenital malformations and, as a group, are one of the most important causes of intractable epilepsy that is surgically remediable.
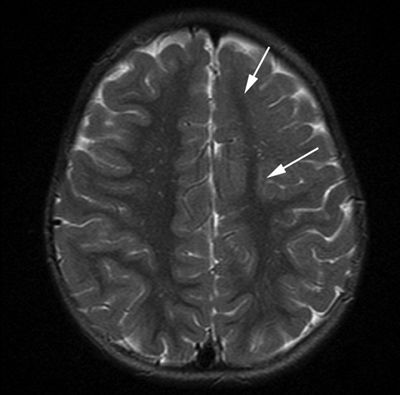
Figure 5.11. Lobar cortical dysplasia. Axial T2-weighted image from MRI without gadolinium in a 3-year-old with infantile spasms shows generalized blurring of the gray–white interface with lack of normal white matter arborization in the left frontal lobe (arrows).
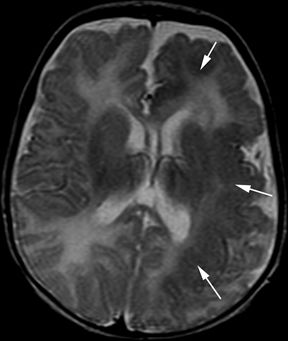
Figure 5.12. Hemispheric malformation of cortical development. Axial T2-weighted image from MRI without gadolinium in a 4-year-old boy with intractable infantile spasms since birth shows diffuse left hemispheric cortical thickening with lack of normal arborization of white matter (arrows).
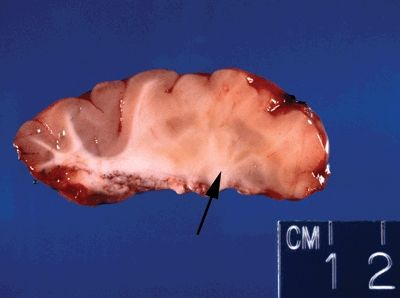
Figure 5.13. Gross appearance of cortical dysplasia marked by an indistinct gray/white interface (right portion of cross section—arrow) with evidence of gray matter tissue abnormally placed in white matter (nodular heterotopia).
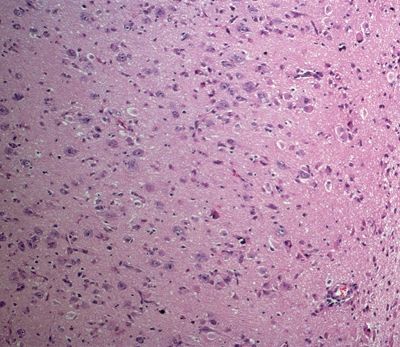
Figure 5.14. Histologic appearance of cortical dysplasia marked by a loss of normal cortical lamination, increased cellularity, and malpositioning of neurons within the cortex. Neurons normally have their apical dendrites oriented perpendicular with respect to the surface of the brain.
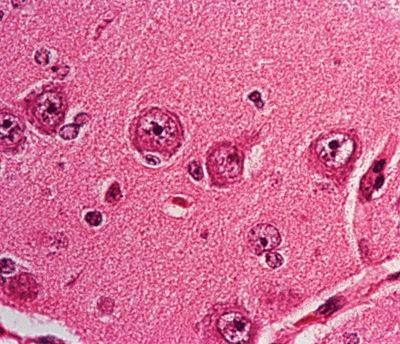
Figure 5.15. High-magnification appearance of neurons in cortical layer II of the parietal lobe in a patient with cortical dysplasia. The neurons are abnormally enlarged in size (neuronal cytomegaly) without any other evidence of dysmorphic features.
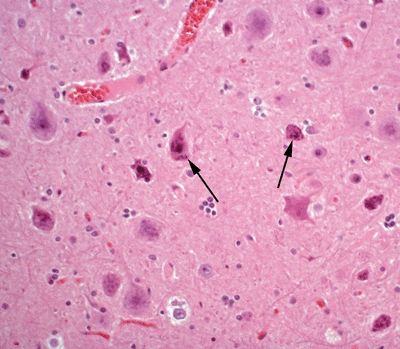
Figure 5.16. Histologic appearance of neurons in cortical layer III of the temporal lobe in a patient with cortical dysplasia. The neurons are marked by abnormal cytologic appearance (dysmorphic neurons) (arrows), including abnormal nuclear morphology and atypical distribution of Nissl substance. In addition, neurons are haphazardly arranged within the cortex.
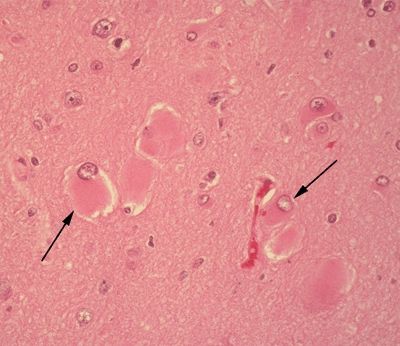
Figure 5.17. Histologic appearance of balloon cells (arrows) in the setting of cortical dysplasia. Balloon cells are marked histologically by the presence of abundant eosinophilic cytoplasm and eccentrically placed nuclei. Multinucleation may be observed. The derivation of these cells is still debated. A subset of balloon cells stain with markers of both glial differentiation (glial fibrillary acidic protein) and neural differentiation (neuron-specific enolase).
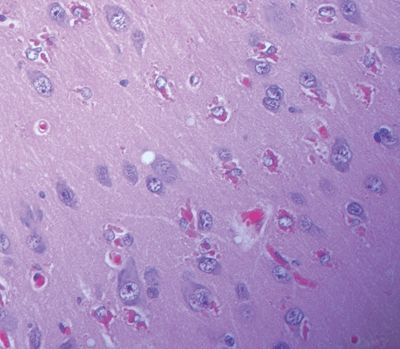
Figure 5.18. Hyaline protoplasmic astrocytopathy of the neocortex may be seen in cases of focal cortical dysplasia. This lesion is marked by eosinophilic inclusions in the cytoplasm of protoplasmic astrocytes in the neocortex, representing a filaminopathy.
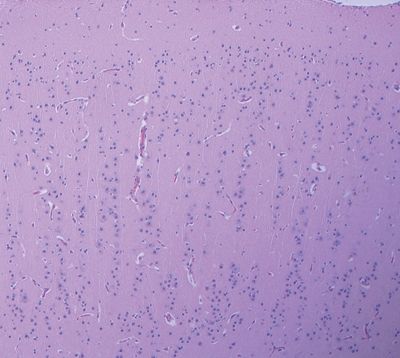
Figure 5.19. Focal cortical dysplasia marked by a linear array or columnar arrangement of cortical neurons. This pattern may rarely occur in isolation or more frequently occurs in conjunction with disorganization in the horizontal orientation in the cortical architecture.
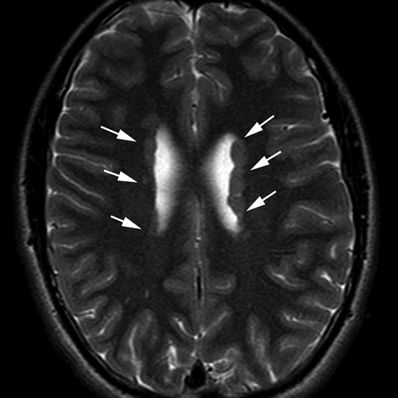
Figure 5.20. Subependymal (periventricular) heterotopia. Axial T2-weighted image from MRI without gadolinium in a 14-year-old female with history of ptosis and tremors shows gray matter nodularity lining the lateral ventricles bilaterally (arrows). Bilateral periventricular nodular heterotopica could be an X-linked dominant condition due to filamin A gene mutations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








