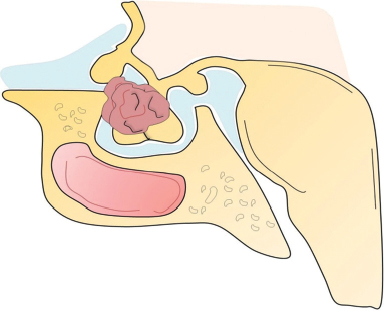
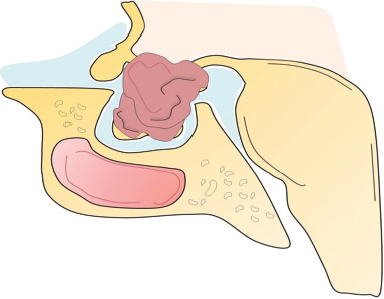
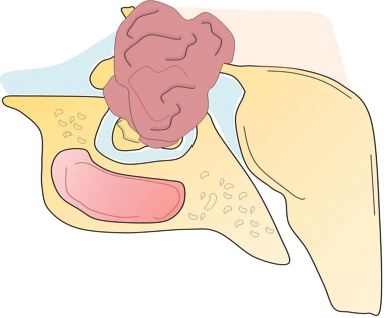
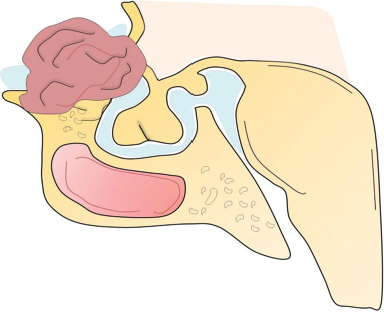
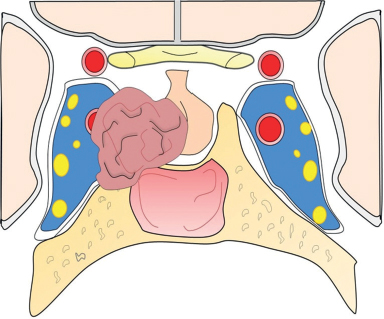
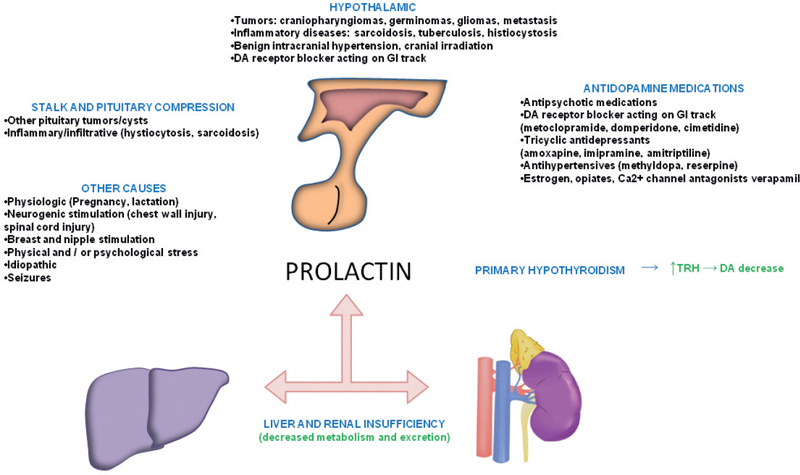
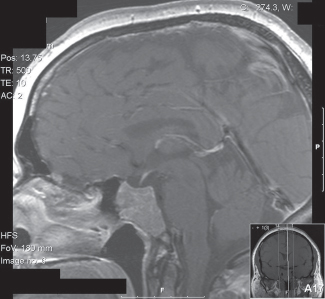
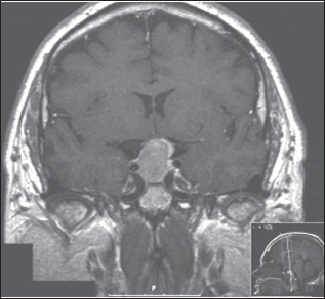
41 What is the prevalence of pituitary tumors? 16.7% (14.4% in autopsy studies and 22.5% in radiologic studies)1,2,3 What percentage of all intracranial neoplasms are pituitary lesions? Approximately 15% (10 to 25%, more common in autopsy series)1,4 Are pituitary tumors more common in men or women? Neither. The male/female ratio is approximately 1:1. In what age group do pituitary tumors most commonly present? 30s to 40s Are there specific types of pituitary tumors more common in males? Somatotrophs (male/female ratio of 2:1) Are there specific types more common in females? Lactotrophs and corticotrophs (male/female ratio of 1:4) How do pituitary tumors present clinically? • Endocrine pathology (over– or underproduction of pituitary hormones) • Mass effect (e.g., bitemporal hemianopsia due to compression of the optic chiasm, hydrocephalus, cranial nerve palsies) • Incidental finding5 What is the differential diagnosis of a sellar lesion? Tumors 1. Pituitary adenoma 2. Pituitary carcinoma 3. Craniopharyngioma 4. Mixed adenoma-gangliocytoma 5. Meningioma6 6. Chordoma 8. Neurohypophyseal glioma 9. Granular cell tumor 10. Germ cell tumor Cystic lesions 1. Rathke’s cleft cyst 2. Dermoid/epidermoid cyst 3. Arachnoid cyst Empty sella syndrome Vascular lesions 1. Saccular aneurysm 2. Cavernous malformation Inflammatory/infectious disease 1. Lymphocytic hypophysitis 2. Granulomatous hypophysitis What is the mnemonic to help remember the differential for suprasellar masses? SATCHMOE S: Sellar neoplasm (e.g., pituitary adenoma), sarcoid A: Aneurysm, arachnoid cyst T: Teratoid lesion (dermoid/epidermoid cyst, germ cell tumor) C: Craniopharyngioma, cleft cyst (i.e., Rathke’s), chordoma H: Hypothalamic glioma, hamartoma, histiocytosis M: Metastasis, meningioma O: Optic nerve glioma E: Eosinophilic granuloma Fig. 41.1 Sagittal T1-weighted MRI with contrast showing densely enhancing sellar/parasellar mass with erosion/involvement of the clivus suspicious for chordoma. Which suprasellar masses are more common in children? Craniopharyngioma (most common), dermoid/epidermoid, hypothalamic hamartoma What are the most common pituitary adenoma subtypes? Lactotroph: prolactin producing Corticotroph: ACTH producing Somatotroph: GH producing Thyrotroph: TSH producing Gonadotroph: FSH/LH producing Plurihormonal Null cell Why is it important to know the subtype of a pituitary adenoma prior to operating? Certain types (e.g., prolactinoma) require medical therapy prior to consideration of surgery What percentage of all pituitary adenomas are lactotrophs (prolactin producing)? Type Prevalence Lactotroph 30% Somatotroph 15% Corticotroph 10% Gonadotroph 10% Plurihormonal 9% Thyrotroph 1% Null cell 25% What are the three biological classifications for secretory pituitary adenomas? 1. Benign adenoma 2. Invasive adenoma 3. Carcinoma Describe the anatomical classification of pituitary adenomas. Originally devised by Jules Hardy.15 Grade: 0—microadenoma (<1 cm); intrapituitary with normal sellar floor 1—microadenoma (<1 cm); normal-size sella with asymmetric sellar floor 2—macroadenoma (>1 cm); enlarged sella with intact floor 3—macroadenoma with enlargement or invasion of floor or suprasellar extension 4—macroadenoma with destruction of sella The Hardy classification was modified by Charles Wilson to include what factor(s)? Extrasellar expansion affecting the ventricles or parasellar space16 Class A Fig. 41.2 Tumor bulges into chiasmatic cistern. Class B Fig. 41.3 Tumor reaches floor of the third ventricle. Fig. 41.4 Tumor extends into the third ventricle to the foramen of Monro. Class D Fig. 41.5 Tumor extends into the middle or anterior fossa. Fig. 41.6 Extradural spread (extends into or out of cavernous sinus). Invasion into which anatomical structure is a poor prognostic factor for surgical outcome? The cavernous sinus On which factors is the histological classification of pituitary adenomas based? Immunocytochemical: stains for hormonal production Ultrastructural: confirms nonfunctioning tumors of pituitary origin Describe the functional classification of pituitary adenomas. Characterized according to hormonal production and associated clinical sequelae: Lactotroph: prolactin producing Corticotroph: ACTH producing Somatotroph: GH producing Thyrotroph: TSH producing Gonadotroph: FSH/LH producing Plurihormonal Null cell Why are pituitary tumors no longer classified according to tinctorial stain? Poor correlation among staining, hormone production, and cell derivation What are the three histological stain groups? Chromophobic, basophilic, and acidophilic ACTH producing, TSH producing, and FSH/LH producing Which tumors are acidophilic? GH producing and prolactin producing Which tumors are chromophobic? Null cell tumors and some prolactin-producing tumors What are the characteristic immunohistochemistry stains for each pituitary adenoma subtype?17 1. Prolactinoma a. Densely granulated b. Sparsely granulated 1. + Prolactin (PRL), Pit-1, estrogen receptor (ER) a. + PRL (cytoplasmic) b. + PRL (paranuclear) 2. Somatotroph 2. + Growth hormone (GH), anticytokeratin (CAM 5.2) 3. Corticotroph 3. + Phosphor-Akt substrate (PAS), adrenocorticotropic hormone (ACTH), proopiomelanocortin (POMC) peptides (e.g., β-endorphin, α-MSH, β-lipoprotein) 4. Thyrotroph 4. + PAS, β-thyroid-stimulating hormone (TSH), α-subunit 5. Gonadotroph 5. + β-follicle-stimulating hormone (FSH) and/or β-luteinizing hormone (LH), α-subunit 6. Null cell 6. ± β-FSH/LH, α-subunit What are the important histological structures seen in each type?18 1. Prolactinoma a. Post-dopamine agonist Rx 1. Central, oval nuclei; “Golgi pattern” PRL stain a. Hyperchromasia of nuclei, perivascular/interstitial fibrosis 2. Somatotroph a. Densely granulated b. Sparsely granulated 2. a. Central nucleus, prominent nucleoli, considerable granularity, GH+ diffusely b. Eccentric nucleus, “fibrous bodies”—paranuclear eosinophilic structures (stain for cytokeratin), GH+ focal stain in paranuclear pattern 3. Corticotroph 3. Large nuclei, coarse chromatin, papillary/sinusoidal pattern, Crooke’s hyaline change (hyaline “target-cells”)—absent in Nelson syndrome 4. Thyrotroph 4. Angulated cells and long cytoplasmic processes 5. Gonadotroph 5. Papillary pattern, perivascular pseudorosette-like cytoplasmic processes 6. Null cell 6. Chromophobic, diffuse or papillary pattern What genetic/proliferative markers and histological classification predict clinically aggressive behavior in pituitary adenomas? • MIB-1 (Ki-67 antigen) • p53 • Proliferating cell nuclear antigen (PCNA) • Sparsely granulated somatotroph adenomas What are the two primary genes involved in nonfamilial pituitary adenoma tumorigenesis? 1. Gsp (gene coding for a persistently active Gs α-subunit, seen in somatotrophs) 2. PTTG (pituitary tumor–transforming gene)18–21) What are some familial syndromes associated with pituitary adenomas, and what are their associated genetic abnormalities?19 • Pituitary adenoma predisposition (PAP) → AIP (aryl hydrocarbon receptor-interacting protein) • Isolated familial somatotrophinomas (IFS) → loss of heterozygosity at 11q13 • Familial isolated pituitary adenomas (FIPA) → unknown What are some other non–pituitary-specific hereditary conditions associated with pituitary adenomas?19 • Multiple endocrine neoplasia → (MEN-1) • Carney complex → (PRKAR1A) • McCune-Albright syndrome → (GNAS1) What is the most common type of functioning pituitary adenoma? Prolactinomas. They represent 30% of all pituitary adenomas.14 What are prominent signs and symptoms of prolactinomas? Hyperprolactinemia leading to: • Infertility • Sexual dysfunction • Oligo/amenorrhea and galactorrhea in females In which patient population are prolactinomas most common? Females of child-bearing age.14 What is the mechanism of amenorrhea/oligomenorrhea in hyperprolactinemic females? Gonadotropin-releasing hormone (GnRH) secretion from the hypothalamus is impaired. Amenorrhea can be corrected by exogenous GnRH administration. Occasionally large prolactinomas cause hypogonadism due to compression of gonadotrophs as part of panhypopituitarism.17 How is prolactin secretion regulated? Prolactin is secreted via a neurohormonal reflex from lactotrophic cells of the anterior pituitary. Hypothalamic dopamine inhibits prolactin secretion from these cells via D2 receptors. Prolactin activates these dopaminergic neurons in the hypothalamus via a “short loop feedback” system.22 How can primary hypothyroidism lead to hyperprolactinemia? Thyrotropin-releasing hormone (TRH) induces prolactin secretion by suppressing dopamine secretion from the hypothalamus.23 What are some common causes of hyperprolactinemia other than prolactinoma? What is the normal serum prolactin level? In most laboratories, the normal range in males is 5 to 20 μg/L and in females 5 to 25 μg/L.24 How high are prolactin levels in prolactinomas? Prolactin levels are proportional to tumor size. Commonly levels are >250 μg/L and can be up to 10,000 μg/L or even higher. If the result is lower (<200 ng/mL) and prolactinoma is suspected, what should be done next? Repeat the test at 1:10 and 1:100 dilutions to determine if the actual concentration is higher, but masked by the “hook effect,” for which dilution corrects. What is the “stalk effect”? Elevated prolactin levels secondary to pituitary stalk compression (e.g., by masses, other pathologies). The pituitary stalk delivers dopamine from the hypothalamus to the adenohypophysis. The secretion of prolactin is under natural inhibition from this dopamine. What is the first step in evaluation of hyperprolactinemia? Rule out physiological causes. A complete history and physical examination to assess for pregnancy, drug-induced hyperprolactinemia, neurogenic causes, hypothyroidism, hepatic, or renal disease. What if a hyperprolactinemic patient is taking a drug that is known to cause hyperprolactinemia? Discontinue drug for 72 hours after consultation with prescribing physician (often a psychiatrist) to ascertain safety, and then repeat levels.6 If the drug cannot be stopped, proceed with MRI to rule out pituitary lesion. Should all prolactinomas be treated? No. Only macroprolactinomas and microprolactinomas with bothersome symptoms related to hyperprolactinemia should be treated.25 For example, galactorrhea secondary to hyperprolactinemia from a microprolactinoma may be considered tolerable by the patient, and in this case the microprolactinoma should be monitored but left untreated.26 What are the risks of tumor growth in cases of untreated asymptomatic microprolactinoma? Low. Small increases occur in 7 to 20% of patients. Patients with asymptomatic microprolactinoma should be monitored closely for symptom development (serial examination), changes in prolactin levels (serial serum prolactin measurements), and changes in tumor size (serial MRI).24 What is the first-line treatment for prolactinomas that require treatment (macroprolactinomas and symptomatic microprolactinomas)? Dopamine (DA) agonists (bromocriptine or cabergoline),25,27 which aim to normalize prolactin levels and reduce tumor size. What are some of the side effects of dopamine (DA) agonists? Common: Headache, postural hypotension, nasal congestion, constipation Infrequent: Fatigue, anxiety, depression, alcohol intolerance Rare: Cold-sensitive vasospasm, psychosis Possible: Cardiac valve abnormalities. Cabergoline is often better tolerated than bromocriptine due to its fewer side effects, and it is more effective at normalizing prolactin levels. Cabergoline also has been shown to be effective in some patients who fail to respond to bromocriptine. Bromocriptine, however, is less costly and should be considered a first-line agent in medical settings with limited budget.28,29 Are DA agonists officially approved for use during pregnancy? No. DA agonists are FDA category B status in pregnancy (animal research failed to show teratogenicity but human data are lacking). However, they are often used to prevent growth of a preexisting prolactinoma during gestation. DA agonist treatment should be resumed. When is transsphenoidal surgery indicated for prolactinomas? • Patients who do not respond to, or cannot tolerate, DA agonist treatment • Pituitary apoplexy (hemorrhage in adenoma with rapid development of compressive symptoms) • Cystic prolactinomas (lack of DA-responsive cells) causing compressive symptoms • Successful DA agonist treatment causing CSF leak due to rapid tumor shrinkage • Patients with psychiatric conditions in whom DA agonist treatment is contraindicated25 What is the recurrence rate of prolactinoma after surgery? In microprolactinomas the success rate is high (85%) and recurrence is rare. In macroprolactinomas recurrence is high—up to 80%25 When is radiotherapy indicated for prolactinoma treatment? Patients who have medically resistant tumors or who cannot tolerate medical or surgical treatment What are the major side effects of radiation treatment? Pituitary dysfunction, damage to optic chiasm, radiation necrosis in temporal lobe, secondary tumors (rare) What kinds of laboratories are required for preoperative workup? Both baseline and dynamic hormone levels Which baseline hormone laboratories should be assessed universally? Prolactin, TSH, free T4, ACTH, cortisol, 24-hour urine free cortisol, LH, FSH, estradiol (in women), testosterone (in men), insulin-like growth factor (IGF-I), α-subunit. Fasting blood glucose and a pregnancy test should be assessed as well. When should additional laboratories be ordered? If values are elevated or equivocal Which dynamic tests are useful? • Dexamethasone (Decadron) suppression test (see Cushing’s Disease [Chapter 40, section 40.3]) • Thyrotropin-releasing hormone (TRH) stimulation test (for some prolactinomas) What are the important questions to ask? Ask about endocrinological changes (weight loss/gain, lactation, menstrual cycle changes, peripheral edema, mood swings, temperature changes, etc.). Ask about changes in vision (with reading, driving, blurred vision, diplopia). What are three ways in which pituitary tumors cause symptoms? 1. Hypersecretion of one or more hormones 2. Mass effect 3. Pituitary insufficiency What are some common symptoms or pathologies associated with mass effect? 1. Headache 2. Visual loss 3. Hydrocephalus Are there more specific changes that are associated with different types of tumors? Yes, each type of adenoma provides a unique set of signs/symptoms that permit presumptive diagnosis by history and physical exam alone. What are some of the signs/symptoms of a lactotroph? • Oligomenorrhea or amenorrhea • Reduced fertility • Loss of libido • Erectile dysfunction • Galactorrhea in the estrogen-primed female breast What are some of the signs/symptoms of a corticotroph? • Proximal myopathy • Centripetal fat distribution • Neuropsychiatric symptoms • Striae • Easy bruising • Skin thinning • Hirsutism • Osteopenia What are some of the signs/symptoms of a somatotroph? • Growth of hands and feet • Coarsening of facial features • Peripheral nerve entrapment syndromes • Snoring and obstructive sleep apnea • Jaw growth and prognathism • Osteoarthritis and arthralgia • Excessive sweating • Acanthosis nigricans\ What are some of the signs/symptoms of a thyrotroph? • Palpitations • Tremor • Weight loss • Insomnia • Hyperdefecation • Sweating What are some of the signs/symptoms of a nonfunctioning adenoma? • Pituitary insufficiency, which is due to compression of the pituitary stalk and/or normal pituitary tissue by the tumor; predominantly manifests as secondary hypogonadism • Rarely: ovarian overstimulation, testicular enlargement, or increased testosterone levels What are some of the signs/symptoms associated with a craniopharyngioma? • Panhypopituitarism • Loss of vision • Diabetes insipidus (occasionally) What symptom would you expect to find in the past medical history (PMH) of a pediatric patient with a hypothalamic hamartoma (HH)? Seizures Which type of seizure is characteristic of HH? Gelastic seizures (forced bouts of emotionless laughter) What signs/symptoms are indications for surgery? Visual loss or pituitary dysfunction due to tumor/hemorrhage/necrosis/compression Hypersecretion of ACTH, GH, or TSH Failure of, or intolerance to, dopamine agonists (in prolactinoma) Which imaging modalities have historically been used to evaluate the sella? Plain lateral and pluridirectional skull x-rays and pneumoencephalography If a patient is unable to have an MRI, what imaging technique can be added to CT to further define parasellar involvement? CT cisternography Why are CT images sometimes useful to obtain? They depict: • Bony anatomy and erosions • Intralesional calcifications • Nasal mucosa • Sphenoid shape and pneumatization • Thickness of the sellar floor Which studies are included in a standard “sella protocol” MRI? MRI with and without contrast including: • T1-weighted imaging (T1WI) axial, sagittal, and coronal sections • T2-weighted imaging (T2WI) coronal sections Which sequence is used further to identify microadenomas? Dynamic T1WI with fast-spin echo (FSE) What is the average height of the pituitary gland in adults? 4.2 mm in women 3.5 mm in men In what scenario could a larger pituitary still be considered normal? Pregnancy, hypothyroidism Which MRI sequence is most sensitive for revealing normal sellar anatomy? T1WI30 Which sequences are the most sensitive and specific for detecting pituitary lesions? Thin-cut coronal views with timed contrast (pituitary protocol) How does the normal pituitary appear on MRI? Anterior lobe: similar to white matter on all sequences Posterior lobe: hyperintense on T1WI Pituitary stalk: hypointense (relative to optic chiasm and neurohypophysis) on T1WI Which normal structures enhance with gadolinium contrast? • Anterior pituitary gland and pituitary stalk • Cavernous sinus Do the intracavernous carotid arteries enhance with gadolinium? No (as opposed to CT with contrast, fast-flowing blood appears as a flow void on MRI with contrast) What are some of the important structures to identify for preoperative planning? • Optic chiasm • Cavernous sinus • Internal carotid artery (ICA)/anterior cerebral artery (ACA)/basilar artery • Sphenoid sinus (amount of pneumatization present, location of the septum with relation to the midline) • Clivus • Pituitary gland and other sellar structures (diaphragma sella, bony floor of the sella) • Pituitary stalk How do macroadenomas appear on MRI? • Isointense to gray matter on T1WI • Isointense or hyperintense (heterogeneous) on T2WI • Cystic/necrotic/hemorrhagic components with fluid-fluid levels • Diffuse enhancement with gadolinium • Hyperintense to brain on fluid attenuation inversion recovery (FLAIR) MRI sequence • T2WI gradient echo (GRE) sequence: hemorrhage may be seen Fig. 41.8 Coronal T1-weighted MRI with contrast showing pituitary adenoma with suprasellar extension.
Pituitary Surgery
41.1 Pituitary Tumors
41.2 Preoperative Assessment
41.3 History and Physical Examination Pearls
41.4 Neuroimaging Pearls
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pituitary Surgery
Only gold members can continue reading. Log In or Register to continue