Chapter 107 Positive Airway Pressure Treatment for Obstructive Sleep Apnea–Hypopnea Syndrome
Abstract
Nasal CPAP therapy for obstructive sleep apnea in adults was first described in 1981,1 but it was not until about 1985 that it began to be more widely recognized as a clinically realistic form of long-term therapy. Although patients at that time needed to use an adhesive to attach the mask, the prompt relief of sleepiness led to an increasing number of users, and subsequently technical improvements were made in the design of the masks and pressure-delivery systems. Over the past 10 years, the evidence supporting the use of CPAP has improved both in quantity and quality, driven by the demands of government funding authorities and health maintenance organizations and by the availability of industry sponsorship with the increasing commercial success of the CPAP equipment.2
CPAP is the gold standard of treatment for moderate to severe OSA; it may also be appropriate treatment for mild but symptomatic cases. However, many patients do not use it, or they use it irregularly. Comparative, intention-to-treat trials involving all degrees of severity of OSAH are needed to delineate therapy pathways and should focus on comparative treatments and how to obtain better acceptance of and adherence to treatment. The available data suggest that viable pharmacologic therapy for sleep apnea3–4 will not appear in the near future.
Nasal Continuous Positive Airway Pressure
Mode of Action
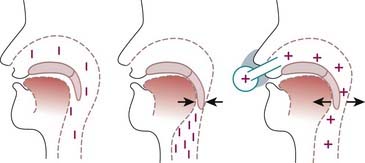
The original experiments with CPAP followed from the concept that closure of the oropharynx in OSAH syndrome results from an imbalance of the forces5 that normally keep the upper airway open (see Chapter 101). In the first description of CPAP use for treatment of OSAH in 1981,1 it was suggested that nasal CPAP acts as a pneumatic splint to prevent collapse of the pharyngeal airway (Fig. 107-1). This notion has been confirmed by a number of studies that either demonstrated the pneumatic splint by endoscopic or other imaging or that showed that CPAP does not increase upper airway muscle activity by reflex mechanisms.6 Detailed magnetic resonance imaging has confirmed that CPAP increases airway volume and airway area and reduces lateral pharyngeal wall thickness and the upper airway edema that result from chronic vibration and occlusion of the airway.7
In addition, recent data support the role of changes in lung volume increments, whether induced by CPAP or manipulated by experimental methodology (head-out rigid shell with adapted vacuum or blower attachment applying extrathoracic negative or positive pressure to the chest), in contributing to improved measured parameters of sleep-disordered breathing.8,9 It is believed that increasing lung volume enhances upper airway size and promotes stability via a caudal traction effect on the upper airway, and conversely, lung volume decrements provide an opposite effect. This lung volume effect can also contribute to the relatively lower level of PAP pressure required in elderly OSA patients.10
For any positive airway pressure (PAP) device, the apparatus providing the pressure at the nasal airway must have the capacity to maintain the prescribed pressure during inspiration (Fig. 107-2). The simplest CPAP systems involve an air blower with sufficient pressure-flow characteristics to provide CPAP via a fixed resistive leak in the system (typically adjacent to the mask).
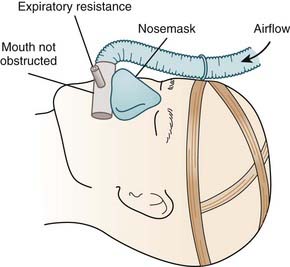
Figure 107-2 Diagram of method of applying continuous positive airway pressure by nose mask.
(From Sullivan CE, Issa FG, Berthon-Jones M, et al. Home treatment of obstructive sleep apnea with continuous positive airway pressure applied through a nosemask. Bull Eur Physiopathol Respir 1984;20:49-54.)
CPAP and Central Apnea
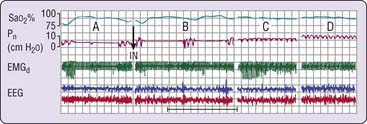
Regardless of the mechanism, nasal CPAP has been documented to be effective in eliminating both obstructive and mixed apneas.11 Some central apneas, particularly those observed in patients with predominantly obstructive events, are also eliminated by nasal CPAP (Fig. 107-3).11 Clearly, some central apneas are associated with increased upper airway resistance, and it could be argued that it is better to consider apnea classification as being CPAP responsive or CPAP nonresponsive. CPAP might also be effective in central apneas associated with cardiac failure on the one hand12,13 but can elicit them in the context of the entity referred to as complex sleep apnea on the other (see later).
Practical Aspects of Treatment
Regardless of the location or method of CPAP titration, there is a need for proper assessment of the patient before initiating CPAP (to determine, for example, whether the patient has awake respiratory failure or marked hypoxemia in sleep), and this requires specific physician training and experience. There is no evidence for the safety and efficacy of CPAP titration outside of a medically supervised process.14 Current evidence supports the use of trained technologists to provide patient education, technical aspects of titration, and follow-up. However, data from small groups of patients has challenged the notion that close medical supervision is mandatory, and this issue should continue as an ongoing major research focus.15 Dominant determinants of CPAP use include the patient’s understanding of the therapy as well as willingness and ability to overcome practical aspects of using CPAP (self-efficacy), its impact on symptoms, and close professional support, regardless of mask type, CPAP manufacturer, and mode of delivery.
The First Night
Sleeping with a nasal or other mask or interface and feeling the pressure sensation of CPAP is not necessarily uncomfortable, but it is a novel experience for the patient. Explanations, demonstration videos, and sessions for acclimating to the mask before beginning CPAP are routine in many centers. Education about CPAP reduces the patient’s anxiety and improves acceptance. Current evidence provides support for the benefit of more intensive patient education in CPAP use.14
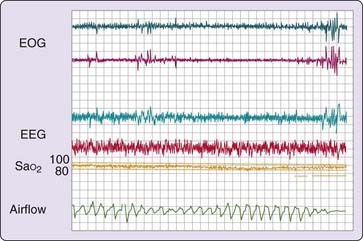
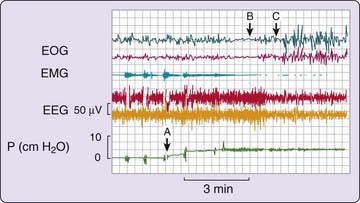
On the first night of treatment, it is important to ensure that the pressure level that is identified as being most therapeutically effective is sufficient to prevent apnea, hypopnea, and associated oxyhemoglobin desaturation as well as respiratory-related arousals in all sleep stages experienced during the titration and in supine and lateral recumbent postures (Fig. 107-4). Thus, simply preventing apnea and hypopnea is not the endpoint of CPAP titration. It must be ensured that the airflow pressure tracing is normal and not chopped off or flow limited, (Fig. 107-5).2 It is important to correct flow limitation, because it can indicate residual upper airway obstruction, potentially causing arousal and disrupting sleep.16
Studies have emphasized the importance of proper airflow measurement during CPAP titration using pressure-flow transducers rather than thermistors or other more indirect airflow measures.17 Proper airflow measurement can help determine the optimal pressure level by providing insights regarding the etiology of arousals—for example, whether they are related to respiratory events (respiratory-related arousals) and whether increasing pressure has a beneficial effect on sleep continuity. Although acute (single-night) studies suggest that correcting inspiratory flow limitations may be the preferred endpoint of CPAP titration, long-term data are sparse.18
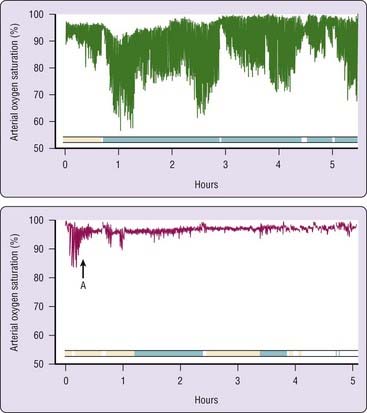
When the correct pressure level is reached and the airway is optimally open, sleep should no longer be fragmented by repeated arousals, and there may be rebound (increased relative proportions) slow-wave and rapid eye movement (REM) sleep (Fig. 107-6).19 This rebound phase of recovery from severe sleep fragmentation lasts about a week, but the duration and intensity of these rebound sleep episodes decrease quickly after the first night of treatment.19 Other factors such as age and medication use might also be relevant determinants of sleep architectural patterns in such CPAP titration sleep studies.
Continued frequent arousals can indicate that a critical level of upper airway resistance persists, especially if the arousals are associated with flow limitation. Persistent snoring is another sign of inadequate CPAP pressure; its specific elimination by titration strategies is supported to some additional degree by preliminary evidence that snoring alone (without the context of defined OHAHS) can contribute to cerebrovascular morbidity.20
Data demonstrate a hysteresis in the relationship between CPAP and upper airway resistance: higher pressures may be required to eliminate inspiratory airflow limitation during upward titration than with downward titration from higher pressures.21 For example, this means that a greater CPAP pressure may be required to render an airway open during the earlier, upward titration stages of a study than is required to maintain airway patency later in the study. An up–down titration protocol (wherein the pressure is initially titrated upward to alleviate obstructive sleep-disordered breathing and then reduced to the lowest level at which such improvement is maintained) can thereby enable a somewhat lower recommended set pressure prescription in patients whose pressure requirement is high. Thus, with an up–down titration strategy for fixed-pressure CPAP, patients may start the night at home with the predetermined pressure and prevent obstructive events from developing at a somewhat lower pressure; while patients may receive an advantage from the hystersis gap, this titration method has not been systematically evaluated.
Considering the length of time CPAP has been used to treat patients with OSAH, the published data on the variability in CPAP pressure requirement is sparse. Some evidence exists for higher pressure requirements with the supine posture22 and in REM sleep,23 and lower pressure in elderly patients.10
It appears that a pressure level accurately set on one night is generally effective on subsequent nights.24 Early research and clinical experience suggested that this was the case, but the use of autotitrating CPAP technology in the home has provided supporting evidence.25 For patients who report continued daytime sleepiness on home treatment despite an initially favorable CPAP titration and subsequent adequate adherence, it may be appropriate, at least as an initial intervention, to empirically increase CPAP pressure under the presumption that the laboratory study underestimated the pressure requirement.
Causes of sleepiness other than OSAH might also need to be considered in this setting. Other factors can have an impact on the therapeutic efficacy of a given CPAP pressure in the home. Weight gain can lead to a need for a higher CPAP setting.26 Heavy (but not moderate) alcohol consumption can affect CPAP pressure, presumably because alcohol depresses upper airway neuromuscular tone.27 Reasonable advice is to avoid all alcohol within a few hours of sleep, but if alcohol is to be taken within a few hours of sleep, the otherwise healthy patient should restrict consumption to modest levels, such as two standard alcohol drinks for men and one for nonpregnant women. It is also important to warn patients of likely adverse effects on sleep apnea of consuming excessive alcohol in the evening. Nasal congestion or a different posture in the home compared to during the CPAP titration can also lead to different pressure requirements, but this has not been well researched.
Treatment of Decompensated Patients with Cardiorespiratory Failure
Previously, the therapy choice for these patients with chronic respiratory failure was tracheal intubation or tracheostomy. Intubation may still be an option; however, in trained hands, nasal CPAP or noninvasive ventilation (see Chapter 113) readily controls the breathing disturbance during sleep. Many of these patients have both upper-airway obstruction and hypoventilation, and nasal CPAP might not be adequate to normalize gas exchange.28 Increasingly, the clinical approach in these patients is to employ bilevel positive pressure therapy.
Autotitrating CPAP approaches are inappropriate in such patients. When possible, hospitalization would be the most reasonable approach in managing patients with severe CO2 retention resulting from a hypoventilation syndrome or chronic lung disease until studies showing the safety of non–hospital-based approaches in these patients are available. Many patients with milder forms of obesity hypoventilation syndrome may be able to be adequately titrated in a supervised laboratory setting with CPAP rather than with more complicated forms of ventilation.29 For patients with mild obesity hypoventilation syndrome this implies a facility to switch to an alternative form of ventilatory support (such as bilevel positive pressure) through the titration night when CPAP is inadequate in this setting (20% of patients in reference 29).
CPAP on the Night Sleep Apnea Is Diagnosed? The Split-Night Study
It has been suggested that CPAP treatment may be initiated on the same night the diagnosis is established.30,31 However, in these studies, patient selection was not randomized: split-night studies (with the first part of the night used for diagnosis and the second part for treatment) tended to be performed in patients who had more severe disease and had been waiting a shorter time for CPAP titration. Other studies have identified a subset of patients for whom a split-night study provided insufficient time for CPAP titration to achieve a satisfactory prescription.32 Patients with milder degrees of sleep-disordered breathing (i.e., with an apnea–hypopnea index <20) in whom the titration was initiated later in the night (because prolonged monitoring was required to establish a diagnosis) were more likely to have unsuccessful split-night titrations.
The American Academy of Sleep Medicine Positive Airway Pressure Titration Task Force has recognized the advantages and some limitations of split-night studies and has recommended (in a guideline) that such studies follow a titration algorithm similar to that for full-night titration studies.33 Thus, in brief, both full-night and split-night titration studies involve upward titration of CPAP pressure at increments of at least 1 cm of water over periods of at least 5 minutes according to the presence of obstructive breathing events and until a period of at least 30 minutes without such events is observed, to a maximum recommended pressure in adults of 20 cm H2O. Obviously, in a split-night study where the first 2 hours or more is used for diagnostic purposes, there is less time available to complete the titration process. In clinic populations of patients with high prevalence of OSAH symptoms and moderate to severe disease, the cost effectiveness of split-night studies has been demonstrated in modeled analyses.34,35
CPAP titration potentially can be accomplished during the day, but there have been few published studies of this approach.36 In one study, both daytime and nocturnal CPAP titration studies yielded sufficient amounts of REM and non-REM (NREM) sleep to help determine CPAP settings. The diurnal and nocturnal CPAP titrations resulted in comparable therapeutic pressures, resolution of sleep-disordered breathing, and adherence for 1 week.
Commencement of CPAP at Home?
Theoretically, it may be economically advantageous to start the first night of CPAP application at home and avoid altogether in-laboratory polysomnographic CPAP titration, but outcome studies showing true cost saving are not available. Current reviews and guidelines do not advocate home commencement of CPAP, particularly using autotitrating devices, across the full spectrum of severity of sleep-disordered breathing. It may be a justifiable clinical strategy, however, in patients who have a confirmed diagnosis of symptomatic, moderate to severe OSAH, who have no attendant significant medical or psychiatric comorbidities, who have had appropriate pre-CPAP education, and for whom an appropriate interface (mask) has been selected.37 This approach implies a major change in practice in sleep centers.
A number of studies have shown that an effective CPAP fixed pressure may be derived from data analysis of one or more unattended nights’ recording using autotitrating CPAP; generally, the effective pressure was determined by visual inspection and determination of the 90th percentile pressure from raw airflow data.38–40 One study observed poorer CPAP adherence in patients assessed only with respiratory monitoring,41 but this is not a universal finding.42 Other workers have found reasonable usefulness with unattended in-hospital CPAP titration but not in patients with significant underlying cardiorespiratory disease.43
Studies have shown equivalence of most outcomes up to 6 months after initiation of CPAP whether the set pressure was derived from in-laboratory techician-attended polysomnography (PSG), from unattended autotitrating CPAP for a number of nights with derivation of a set fixed pressure from data analysis of those nights, from autotitrating CPAP as initial and ongoing mode, or from formulaic prescription approaches.40,44–48 Limited advantage for autotitrating CPAP has been seen in a study in which endpoints such as fewer side effects, lower pressures, and patient preference were reported, but without difference in overall compliance or impact on measures of daytime sleepiness or quality of life.49
Other studies have suggested that equations can be determined that would allow an empirical pressure level to be set, potentially obviating costs of pressure-determination studies but also potentially preventing the need for any investigation of CPAP efficacy.32 Recent data support this empirical method of CPAP titration instead of laboratory initiation.15,44 Data have also been presented, however, that demonstrate differences between various formulaic pressure level prescriptions and set pressures derived from autotitrating 95th percentile pressure.50 Predicted pressures based on an artificial neural network may be more accurate than regression equations.51 Long-term outcome data for this approach are lacking.
Problems and Side Effects
Side effects reported by the patient are usually, but not exclusively, related to pressure or airflow or the mask–nose interface (Box 107-1). Side effects are important because patients with complaints in this regard use CPAP less often than those without side effects.52 A nonspecific sense of claustrophobia may be reported by patients, but this often involves mask or interface problems, nasal congestion, or exhalation difficulties. Dangerous complications of nasal CPAP therapy (e.g., pulmonary barotrauma, pneumocephalus, increased intraocular pressure, tympanic membrane rupture, massive epistaxis, and subcutaneous emphysema after facial trauma) are extremely rare and represent isolated case reports in the literature.53
There are occasional case reports of peripheral edema developing after institution of CPAP.54,55 Mycobacterium gordonae pneumonia was reported in an immunocompromised kidney transplant patient who inadequately cleaned a home CPAP unit used to treat OSAH.56 Irritating side effects such as aerophagia and musculoskeletal chest discomfort (presumably related to increased lung volumes) have also been reported.53 Caution should be used when implementing CPAP therapy after neurosurgery or facial surgery or injury.
Nasal Congestion
Nasal congestion is a common side effect of CPAP therapy.53 Although most patients experience initial self-limiting nasal congestion, at least 10% complain of persistent nasal stuffiness to some degree after 6 months of therapy.57
There appear to be many reasons for nasal symptoms. CPAP can provoke pressure-sensitive mucosal receptors, leading to vasodilation and mucus production. In some patients, it can unmask allergic rhinitis by restoring the nasal route of breathing after years of mouth breathing. In others, administration of positive airway pressure in the presence of fixed nasal obstruction with polyps or a deviated septum can produce symptoms. Mouth leaks also cause increased nasal resistance, probably by exposing the nasal mucosa to higher flows and reduced relative humidity.58
Treatment of nasal congestion depends on the cause. Mouth leak may be minimized if an incorrectly high CPAP pressure is down-adjusted. Sometimes it is necessary to use a chin strap to minimize mouth leak. These are often uncomfortable, and acclimation to this accessory is often necessary. Heated, rather than cold, pass-over humidification is best suited to increase the relative humidity and may be used to treat nasal congestion.58,59 Nasal congestion can be treated with antihistamines, topical steroids, or topical saline sprays, and heated humidification of the circuit improves nasal, mouth and throat dryness.60 Intranasal ipratropium bromide can be helpful in abating CPAP-induced rhinorrhea. However, there is no evidence for the routine use of humidification to improve CPAP adherence.61
Nasopharyngoscopy should be performed in patients with persistent symptoms of nasal congestion or those with obvious nasal obstruction, and these patients might require corrective surgery for an obstructive lesion such as a polyp, marked mucosal thickening, or deviated septum. There is some limited evidence of benefit from such intervention in improving CPAP compliance.62–64 Estimating nasal resistance or minimal cross-sectional area before initiating CPAP can identify OSA patients at increased risk of difficulties using CPAP.65 Full-face (oronasal) or oral masks may also be of value in managing patients with nasal side effects.66
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree