For those with peri-ictal depression, improved seizure control may be a sufficient treatment. For patients with resistant peri-ictal depression, interictal MDD, persistent depressive disorder, or IDD, antidepressant treatment is indicated. Antidepressant medications tend to be less effective, however, for peri-ictal episodes. In the absence of controlled trials, the choice of antidepressant should be based upon safety, tolerability, and ease of use (e.g., frequency of dosage, likelihood of drug–drug interactions). If a particular antidepressant was successful in the past for the patient or a family member, another trial of this agent should be considered.
A common misconception is that all antidepressants significantly lower seizure threshold and should be avoided. These fears are largely based upon seizures associated with overdoses, which have little predictive value when levels are within therapeutic range (37,38). Lower doses of antidepressants may in fact have anticonvulsant properties (39). Rate of escalation and duration of treatment may also play a role. Patients with primary generalized epilepsy may have a greater propensity for seizure exacerbation secondary to antidepressants; depression in such patients appears to respond well to low doses of these agents (16).
The medications with substantial risk are few; however, it is prudent to avoid bupropion, maprotiline, clomipramine, and amoxapine because of their potential for exacerbating seizures (39). Seizures due to bupropion are classically generalized tonic–clonic convulsions (GTC), as may be seen particularly in patients with bulimia. The immediate-release preparation presents the greatest concern, with a seizure incidence of 0.36% to 5.8%. The seizure-inducing potential is dose related, and the therapeutic index is low. Maprotiline induces seizures in 12.2% to 15.6% of patients; higher serum levels and longer durations of treatment are the risk factors. The epileptogenicity of clomipramine varies by dose, with seizures in up to 3% of patients taking >250 mg/day. Risk also increases with concomitant VPA, with status epilepticus occurring in some cases. Although the propensity for seizures is lower (0.5%) with doses <250 mg/day, clomipramine is best avoided. Likewise, seizure risk with amoxapine is 36.4%, with reports of status epilepticus.
In contrast, selective serotonin reuptake inhibitors (SSRIs; citalopram, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline) are unlikely to worsen seizure frequency or severity and are generally effective for dysthymic disorders, symptoms of irritability, and poor frustration tolerance. Furthermore, an overdose of an SSRI is unlikely to be fatal, interactions with AEDs are minimal, and side effects are manageable. For these reasons, SSRIs are first-line treatments in adults and children with depressive disorders. Women tend to be more responsive than men, however, and sexual dysfunction and weight gain are common adverse reactions. Sexual dysfunction may occur in 70% of those treated with SSRIs. Weight gain is of particular concern when SSRIs are used in combination with AEDs that cause the same effect, including gabapentin, VPA, CBZ, and pregabalin.
Among the SSRIs, sertraline has been best studied. Kanner et al. (17) used sertraline to treat depression in 100 patients with epilepsy. Depressive symptoms improved in the majority of subjects, with seizures definitely worsening in only one patient. Clinicians favor the use of the newer SSRIs, citalopram and escitalopram, due to their lack of hepatic enzyme effects. Fluoxetine and fluvoxamine, in contrast, have enzyme-inhibiting effects that may elevate AED levels. Starting with the lowest dose of an SSRI is recommended, with a gradual dose increase at 1- to 2-week intervals. If a more rapid increase is necessary due to severe symptoms, closer observation is required.
Vortioxetine, approved in 2013, acts as an SSRI, but with additional serotonergic effects, including 5-HT3 receptor antagonism and 5-HT1A receptor agonism. Seizure induction rates have not yet been published, and data in epilepsy populations are lacking. Vortioxetine levels are decreased by CYP inducers, including CBZ and phenytoin (DPH). Vilazodone, a newly approved SSRI and partial serotonin 5-HT1A receptor agonist, has not yet been studied in patients with epilepsy. Prescribing information notes that “seizures can occur with treatment,” although induction rates were not specified. An isolated case of generalized tonic–clonic status epilepticus was reported in a young child with a toxic ingestion (40). Given such limited seizure-related data and overall experience, vortioxetine and vilazodone are unlikely to be prescribed for epilepsy patients at present.
The tricyclic antidepressants (TCAs; amitriptyline, amoxapine, clomipramine, desipramine, doxepin, imipramine, nortriptyline, protriptyline, trimipramine) are not recommended as first-line agents in patients with epilepsy because of a greater likelihood of side effects and drug–drug interactions. Weight gain and sexual dysfunction are common. Also of concern are the potential for cardiac conduction abnormalities and the greater tendency to induce mania. The anticholinergic effects may exacerbate memory dysfunction in patients with Alzheimer disease as well. Finally, these medications have been shown to increase the risk of seizures in the general population in up to 0.1% to 4% at therapeutic levels and 8.4% to 22% in the setting of overdose (levels >1000 mg/mL). This class of medications is contraindicated in children with epilepsy due to the seizure risk. Imipramine and amitriptyline at dosages ≤200 mg/day, however, do not generally provoke seizures in adults. Imipramine, amitriptyline, and trimipramine may cause epileptiform EEG changes at high doses, although this is not generally accompanied by clinical seizures.
For these reasons, monitoring TCA levels may be helpful, particularly in the setting of polypharmacy or to identify slow metabolizers. Enzyme-inducing AEDs (e.g., phenytoin [DPH], PB, primidone, CBZ) may cause low TCA levels, while enzyme inhibitors (i.e., VPA) may increase TCA levels. Conversely, imipramine and nortriptyline may increase concentrations of DPH, CBZ, and PB, and amitriptyline may increase the volume of distribution of VPA. Drug–drug interactions may be quite complex, at times with increased formation of toxic metabolites but decreased activity of parent compounds. The common recommendation to start at a low dose and increase slowly applies.
The monoamine oxidase inhibitors (MAOIs; rasagiline, selegiline, isocarboxazid, phenelzine, tranylcypromine) are generally safe in patients with epilepsy. They are not often prescribed due to their side effect profile, however, which includes hypertensive crises due to interactions with tyramine-containing foods. The potentially fatal serotonin syndrome may also occur when an MAOI is combined with an SSRI or a TCA, with symptoms including restlessness, myoclonus, diaphoresis, tremor, hyperthermia, and seizures. Although useful for atypical features of depression, these are third-line agents and should be prescribed only by psychiatrists.
Of the selective norepinephrine reuptake inhibitors (SNRIs; duloxetine, venlafaxine), venlafaxine is a first-line agent in adults with depression, particularly for those with melancholic features. Dosages as high as 225 mg/day have been demonstrated to be safe in depressed patients with epilepsy. Lethargy, irritability, and hypertension are the main side effects. Blood pressure elevations are typically seen at higher doses, above 300 mg/day.
Levomilnacipran is a new serotonin and norepinephrine reuptake inhibitor, FDA approved in 2013. An isolated case of seizure activity was reported in premarketing clinical studies, but data regarding use in epilepsy patient populations are not yet available. As with many new psychiatric medications, the drug is not commonly used in epilepsy patients, pending further experience and less expensive generic formulations.
The goal of treatment for depression is symptom remission; those with any residual symptoms have a greater likelihood for relapse. A full response should be evident in 6 to 12 weeks. Continuation of medication is generally indicated for 4 to 9 months. The ILAE consensus guidelines recommend continuation of treatment for 6 months after recovery from the initial episode and for at least 2 years after recovery from any subsequent episodes (31). If a patient has three or more episodes of depression, residual symptoms, suicidality, psychosis, or an otherwise severe episode, long-term prophylaxis is indicated. Children tend to have high relapse rates, with continuation of symptoms into adulthood (41).
In the elderly, SSRIs and venlafaxine may be used, but dosages should begin at one-half the usual starting dose, and treatment should be continued for at least 2 years in those with frequent or severe episodes (20).
In addition to pharmacotherapy, evidence supports use of cognitive–behavioral therapy (CBT) and interpersonal therapy in those with mild to moderate symptoms, and additional benefit may be attained from combined approaches with therapy plus medication. Psychotherapy can help patients cope with limitations imposed by epilepsy and may result in significant improvements in rating scales of depression and anxiety, as well as seizure frequency (42). Psychoeducation and therapy (e.g., CBT, interpersonal psychotherapy, or supportive therapy) are strongly recommended for children (43).
For refractory depression, alternative regimens may include dopamine agonists and electroconvulsive therapy (ECT), which is particularly useful for refractory depression or acute, severe episodes (e.g., including suicidality, psychosis). ECT is not contraindicated in epilepsy. Dose reduction of AEDs may be required during a course of ECT, and AEDs should be withheld the morning of a treatment unless there is concern for status epilepticus.
The Epilepsy Foundation’s Mood Disorders Initiative has made the following recommendations regarding treatment of depression in adults with epilepsy (20):
Stage 1: Monotherapy with an SSRI (citalopram, escitalopram), venlafaxine or mirtazapine, and/or CBT is first-line treatment. If there is an incomplete response, proceed to stage 2.
Stage 2: Monotherapy with a different agent is recommended (another SSRI, a TCA, venlafaxine, or mirtazapine). If there is an incomplete response, proceed to stage 3.
Stage 3: Monotherapy with an SSRI, a TCA, venlafaxine, mirtazapine, or an MAOI is recommended. A medication from a different class than that used in stages 1 or 2 should be administered. Alternatively, combination therapy may be used (TCA with SSRI, TCA with venlafaxine, TCA with mirtazapine, venlafaxine with mirtazapine). If there is an incomplete response, proceed to stage 4.
Stage 4: Combination therapy is recommended (TCA with SSRI, TCA with venlafaxine, TCA with mirtazapine, venlafaxine with mirtazapine). If there is an incomplete response, proceed to stage 5.
Stage 5: ECT.
When transitioning between drugs, an overlap and taper strategy should be used to avoid withdrawal symptoms.
The Canadian Network for Mood and Anxiety Treatments (CANMAT) recently issued general recommendations for the treatment of mood disorders in epilepsy (44), which vary somewhat from the Epilepsy Foundation’s guidelines. CANMAT recommendations, for example, suggest lamotrigine as a second-line treatment for depression in epilepsy patients. They also consider the use of folate supplementation, given the concern that AED-associated folate deficiency may contribute to depression. Furthermore, in refractory depression, the authors propose possible VNS implantation.
Suicidality
Though estimates vary, the lifetime prevalence of SI in patients with epilepsy is approximately twice that of the general population, occurring at a rate of about 12%. Suicide attempts occur in 4.6% to 30% of patients with epilepsy, compared to 1.1% to 7% of controls. Patients with seizures are also at greater risk of completing suicide compared to controls: 2.32% to 14% and 0.74% to 1.4%, respectively (45). Elevated risk occurs in children and adolescents with epilepsy as well, with 20% of such children experiencing SI (46).
The prevalence of suicide in epilepsy increases with comorbid psychiatric diagnoses, including depression, psychosis, anxiety, personality disorders, and bipolar disorder (45). Ictal and postictal depression, mania, postictal psychosis (PIP), and command hallucinations present particular risks. In 90% to 95% of patients who commit suicide, prior psychiatric diagnoses were present (45).
Other risk factors include psychosocial stressors, poor physical health, young age in men (25 to 49 years), early age of seizure onset (<18 years, particularly during adolescence), presence of brain lesions, inadequate follow-up or treatment of seizures, access to firearms or other methods of self-harm, and interictal behavioral disorders (i.e., viscosity) (45,47). In TLE, the suicide rate is 25 times higher than in the general population, and a history of epilepsy surgery presents a risk five times of that presented by medical management. Furthermore, cognitive impairment carries a 10 to 25 times greater risk than normal cognition. The degree to which these factors are predictive, however, may differ between men and women (48).
Time periods for particular concern are in the first 6 months after the diagnosis of seizures (49) and within a few months to years of attaining good seizure control after a long history of refractory epilepsy (50). SI may also occur with a temporal relationship to seizure activity. Among patients with refractory seizures, 13% experience postictal SI, lasting 24 hours on average.
Of concern is suicidality associated with AEDs, especially PB (36,48). The risk of SI is 47% in those treated with PB compared to 4% in those treated with CBZ (36). The relationship may be related to dose (48). Patients taking PB should be specifically monitored for the development of SI, and use of the drug should be avoided in those with depression or cognitive dysfunction.
In early 2008, the FDA issued an alert regarding suicidality and use of AEDs (51). Based upon a meta-analysis of 199 placebo-controlled trials including 11 AEDs, they found approximately twice the risk of suicidal thoughts or behavior in those taking AEDs compared to placebo (0.43% vs. 0.22%, respectively). The FDA interpreted the findings as likely representing a class effect, generally consistent across medications. Rates differed, however, between the studied AEDs, and older AEDs were not included in the analysis. The risk began as early as 1 week, and continued to at least 24 weeks, at which time most trials ended. Demographic factors (i.e., age) did not clearly influence risk, although those using the drugs for seizure control had the highest relative risk of suicidality (3.6) when compared to groups taking these agents for other indications. These findings prompted labeling changes. Physicians are encouraged to discuss this issue with their patients and closely monitor those receiving AEDs for onset or worsening of depression.
Assessment should include direct questioning regarding risk factors. Risk for suicide may also be assessed by the suicidality modules of the Mini International Neuropsychiatric Interview (MINI), the Beck Depression Inventory-II (BDI-II), and the Children’s Depression Inventory (CDI) (20,45). Physicians need to document the level of risk, interventions, and plans for monitoring. The patient must be kept safe, including the removal of firearms from the home, and may be provided hospitalization until the SI resolves. The clinician should also consider the patient’s access to AEDs and the potential for overdose. The availability of PB, for example, poses great safety concerns. Antidepressants and psychotherapy are helpful, and referral to a psychiatrist is indicated.
ANXIETY DISORDERS
Anxiety disorders include generalized anxiety disorder (GAD), panic disorder (PD), obsessive–compulsive disorder (OCD), phobias, and posttraumatic stress disorder (PTSD). Studies suggest an increased prevalence of GAD, PD, OCD, and phobias in patients with either partial or primary generalized epilepsy, with prevalence estimates of 3% to 66% in patients with seizures and up to 29% in the general population (2). Symptoms of anxiety may be more severe in patients with localization-related epilepsy (52,53), and recent data suggest a particularly high frequency of anxiety disorders in patients with left MTS (66.7%) (32). The association between anxiety and epilepsy is bidirectional, with the incidence of anxiety increased in the years pre- and postepilepsy diagnosis (4). Patients may also have symptom complexes that overlap defined categories. Anxiety may lead to significant distress, and the presence of anxiety in a depressed patient with epilepsy increases the risk of suicide (45).
Generalized Anxiety Disorder
GAD is characterized by excessive anxiety and worry about many issues, occurring almost daily. Patients with GAD may also experience restlessness, fatigue, poor concentration, irritability, muscle tension, and sleep dysfunction. Anxiety in epilepsy most commonly presents as GAD, seen in an estimated 21% of patients with refractory TLE (54).
Anxiety may occur prior to (preictal), during (ictal), or after (postictal) seizure onset. Preictal anxiety may precede the seizure by hours to days. Ictal anxiety is often described as “fear,” occurring as part of the aura in approximately 15% of patients with partial seizures and 33% of patients with TLE. Ictal fear is more common with medial foci than with lateral regions of onset. It has been suggested that ictal fear may signify well-localized anterior TLE and predict a favorable surgical outcome compared to those without ictal fear. Ictal anxiety may also be present, however, with frontal, cingulate, or other limbic-onset seizures. While some authors suggest that fear lateralizes to the nondominant hemisphere (55), this is not entirely clear. Postictal anxiety occurs in an estimated 45% of those with refractory partial seizures. Symptoms last an average of 24 hours and have been likened to a “psychiatric Todd phenomenon.” Those at greatest risk for postictal anxiety include patients with a psychiatric history (21).
Up to 66% of patients with epilepsy report interictal anxiety. While data are conflicting, interictal anxiety does not necessarily correlate with seizure frequency (56,57), and symptoms may develop paradoxically with seizure freedom or reduction (i.e., postoperatively). A shorter duration (<2 years) of epilepsy may correlate with increased anxiety.
Contributing factors include the unpredictability of seizures, psychosocial difficulties, and iatrogenic effects. More specifically, the use of felbamate, vigabatrin, LTG, or TPM may predispose to anxiety, particularly with rapid titration. The withdrawal of AEDs, such as benzodiazepines or PB, may also precipitate GAD, particularly in those with ictal anxiety. Increased anxiety can occur as a paradoxical reaction to SSRIs as well.
GAD may affect quality of life even more than seizure frequency. Anxiety prior to epilepsy surgery is a marker of poorer postresection psychosocial adjustment, perceived memory function, and health-related quality of life. Hence, the importance of screening should be emphasized to aid in appropriate treatment and presurgical counseling. A number of assessment tools are available, including the State–Trait Anxiety Scale (STAI, revised scale Form Y), Goldberg Depression and Anxiety Scales, the Beck Anxiety Inventory (BAI), the Symptoms Checklist (SCL-90-R), the Hospital Anxiety and Depression Scale, and the Hamilton Anxiety Rating Scale (HAM-A or HARS) (58).
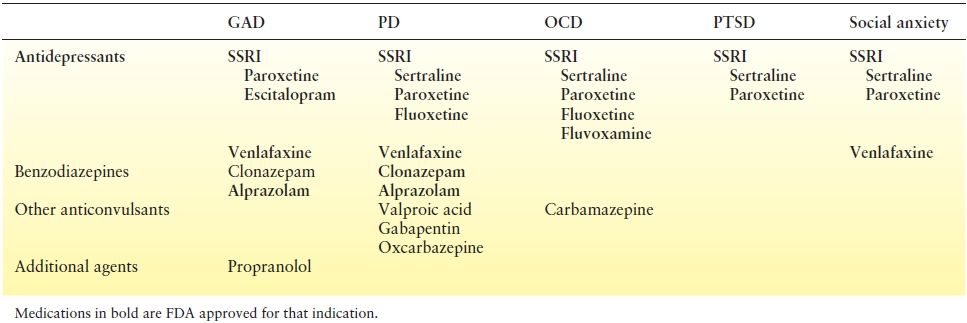
Treatment in patients with epilepsy currently varies little from that of the general population, although no controlled studies have been conducted to date. SSRIs, specifically paroxetine and escitalopram, are first-line agents (Table 93.2). Data also demonstrate efficacy of venlafaxine. Benzodiazepines may be used for insomnia and acute, severe distress, although continuous use should probably be limited due to their addictive properties. Pharmacologic treatments used empirically also include TCAs (i.e., imipramine), trazodone, propranolol, and AEDs. AEDs with anxiolytic effects include VPA, tiagabine, benzodiazepines, barbiturates, gabapentin, pregabalin, phenytoin, oxcarbazepine (OXC), and possibly rufinamide. Mula (59) suggested pregabalin as a first-line agent for acute and long-term maintenance therapy, with paroxetine, imipramine, and venlafaxine as second choices. While buspirone is effective in the general population, this agent should be avoided in patients with epilepsy due to the risk of exacerbating seizures.
Table 93.2 Preferred Agents for the Treatment of Anxiety Disorders
Nonpharmacologic treatment may be helpful in individual cases, including family counseling, supportive psychotherapy, psychoeducational programs, and self-help groups. Although data regarding efficacy are conflicting, CBT may be useful, either adjunctive to anxiolytics or alone, in patients with mild to moderate symptoms. CBT addresses the negative thought patterns that lead to anxiety, followed by desensitization to anxiety-provoking stimuli. In severe cases, anxiety may also be treated by ECT.
Panic Disorder
Panic attacks consist of episodic symptoms including light-headedness, tremor, fear of loss of control or death, paresthesias, shortness of breath, chest pain, palpitations, perspiration, chills, abdominal upset, sensation of choking, derealization, and persistent worry about future attacks. Clinicians must distinguish between seizures manifesting as panic (“ictal panic”), a primary panic disorder (PD), and comorbid epilepsy and PD. Factors favoring the diagnosis of PD include a gradual onset of symptoms, duration from minutes to hours, and lack of postepisode confusion (Table 93.3). Making the distinction, however, may be difficult. Sazgar et al. (60) identified 4.5% of patients with intractable TLE as having been initially misdiagnosed as PD. Mintzer et al. (61) have adapted the MINI with an “Epilepsy Addendum” that attempts to aid in the distinction between PD and ictal fear. Anecdotally, patients often report that they sense the difference between the two types of spells. Still, seizures may be diagnosed only after a long delay, when progression to more clear complex partial events occurs.
Table 93.3 Differentiation of Panic Attacks and Partial Seizures
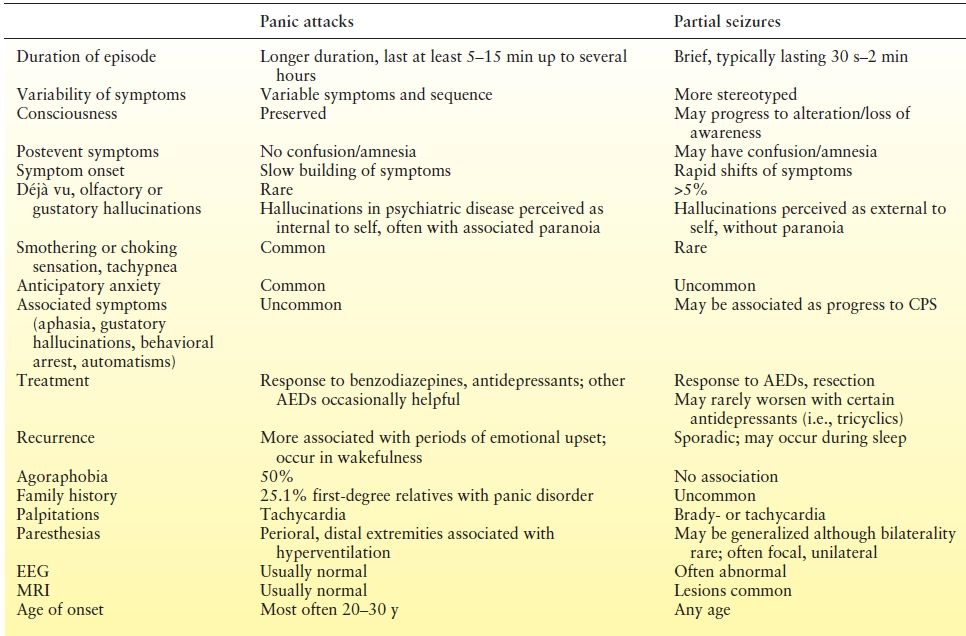
An estimated 21% of patients with epilepsy have comorbid PD (62), in contrast to a prevalence of PD in 1% to 3.5% of the US general population. The comorbidity may occur in up to 33% of patients with ictal fear (61). PD may emerge or worsen after epilepsy surgery, particularly in those with ictal fear. The incidence of PD in epilepsy appears to increase with age.
Seizures manifesting as panic are uncommon. When present, ictal panic is most often associated with right midanterior temporal lobe onset. One study suggested that ictal panic is particularly rare in patients with extratemporal lobe seizures, with no cases observed in a series of 72 such patients (60). Isolated case reports, however, suggest that ictal panic may occur with left parietooccipital lobe– (63), right parietal lobe– (55), and left temporal lobe–onset seizures (64).
Panic attacks may also present as a postictal phenomenon. Like other forms of postictal anxiety, symptoms last 24 hours on average and are predicted by psychiatric history and relatively low seizure frequency.
PD can cause significant distress, and proper treatment should be initiated upon diagnosis. Serotonergic medications and benzodiazepines are the agents of choice (65). FDA-approved medications for the treatment of panic include sertraline, paroxetine, fluoxetine, venlafaxine, clonazepam, and alprazolam. The role for other anticonvulsants in the treatment of PD is unclear, although VPA, gabapentin, and OXC may be helpful. In a recent review, Mula (59) recommended an SSRI and CBT in combination during the acute phase, with a TCA and CBT as a second choice. For long-term maintenance, either SSRI–CBT combination therapy or CBT alone was suggested. Of note, hyperventilation/deep breathing during CBT is contraindicated in patients with PD and epilepsy, per ILAE guidelines (31).
Obsessive–Compulsive Disorder
OCD manifests as obsessive thoughts or repetitive, ritualistic behaviors, typically carried out in order to neutralize anxieties or prevent imagined negative events. The prevalence of OCD in the general population is estimated at 1% to 3%. Limited data suggest an increased frequency of OCD in patients with seizures, with studies demonstrating prevalence between 10% and 22% in patients with TLE (32,66,67). Several case reports also document the co-occurrence of OCD and epilepsy in patients with temporal lobe (68,69), anterior cingulate (70), frontorolandic (71), and primary generalized (72) seizures. Data regarding an association with lateralization of the seizure focus, however, are conflicting. In a study of patients with TLE and IGE, common obsessions included those related to symmetry or exactness, contamination, and aggressiveness, while common compulsions included ordering, washing, and checking (67). Interictal personality characteristics associated with TLE, such as attention to detail or hyperreligiosity, may also be viewed as a mild form of obsessions or compulsions.
OCD in the setting of epilepsy, however, remains underrecognized. In a series of nine patients with TLE meeting criteria for OCD, only one had been previously diagnosed (66). Barbieri et al. (68) described a patient who experienced symptoms of OCD for 17 years and never informed her physicians. These cases underscore the importance of screening and the involvement of neuropsychiatrists in epilepsy clinics. Depression and anxiety are common in patients with epilepsy and obsessive–compulsive symptoms (73), and it is important that these comorbid conditions do not obscure the diagnosis of OCD.
Male sex, older age, earlier age of seizure onset, longer duration of epilepsy, focal-onset seizures, and poor seizure control have been associated with more severe symptoms (73), although the effect of epilepsy duration is controversial. Surgical resection may either ameliorate or worsen symptoms of OCD. Rare cases of postsurgical de novo OCD have also been reported (74).
No controlled trials have evaluated the treatment of OCD in patients with epilepsy, and no consensus regarding management exists. Patients are best managed by an experienced psychiatrist. Idiopathic OCD may be treated with psychotherapy and antidepressants, with SSRIs as first-line medications. When using antidepressants, high doses are often required, with monitoring for seizure exacerbation, side effects, and drug interactions. Although one case report documented a 50% improvement in symptoms, many attempts at nonpharmacologic, behavioral treatments have met with limited success in patients with comorbid seizures (69). Nevertheless, Mula (59) recently recommended CBT as the first choice for acute and long-term treatment in epilepsy patients, with the second choice being CBT with sertraline, followed by CBT with clomipramine, when drug treatment is needed in severe or refractory cases. Successful treatment with CBZ or OXC has also been reported (72). Koopowitz and Berk (72) suggested that comorbid epilepsy may predict better response of OCD symptoms to AEDs than antidepressants, although many other case reports document a lack of effect.
Phobias
Phobias occur in 20% of patients with epilepsy. An estimated 8% to 9% of patients with refractory TLE have agoraphobia and 29% have social phobia (54). Underlying cognitive deficits, low self-esteem, depression, family psychiatric history, and lack of social support may predispose to phobias.
Rare, and perhaps unique to epilepsy, is a “seizure phobia” in which patients fear future seizures. Patients may specifically fear resultant death or brain damage and relive prior seizures. Patients may develop agoraphobia or social phobia, stemming from fear that others would observe their seizures if they were to occur in public. While phobias are typically an interictal phenomenon, some patients experience postictal agoraphobia. The degree of anxiety may parallel the perceived severity of seizures.
Such phobias may be successfully treated by CBT in addition to other forms of counseling and seizure education (75). Caution should be used in the prescription of benzodiazepines, given concerns that they may lead to dependence and avoidance of the deeper cognitive–behavioral issues.
PSYCHOSIS
Epidemiology
The risk of psychosis varies with epilepsy syndrome, seizure severity, and seizure frequency. Incidence rates of psychosis are increased in patients with epilepsy, both before and after the onset of seizures, suggesting a bidirectional relationship (4). Psychosis is reported in 0.6% to 7% of patients with epilepsy in the community and in 19% to 27% of hospital-derived populations (76). The overall frequency of psychosis among patients with epilepsy is approximately 7% to 14%. Most studies indicate a predilection for those with TLE (15.8%), particularly those with left MTS (77,78). Reports of an association with other localization-related epilepsies indicate, less commonly, a relationship with left frontal lobe–onset seizures (79). In addition, a prevalence of 3% to 5% has been documented in patients with IGE.
Diagnosis
Preictal or ictal psychosis is rare. Shukla et al. (80) described a series of patients with intractable right temporal or frontotemporal seizures and preictal psychosis. Symptoms consisted of hallucinations, delusions, affective changes, heightened religiosity, and abusive behavior lasting from 12 hours to 15 days prior to habitual seizures. The psychotic features resolved after each seizure.
During seizures, patients may experience visual or auditory illusions and hallucinations, paranoia, depersonalization, derealization, autoscopy, or a sense of someone lurking behind them. As with most partial seizures, symptoms typically last <3 minutes duration. Prolonged ictal psychosis is rare but may be evident in the setting of nonconvulsive partial or absence status. Often, such patients have a corresponding central nervous system (CNS) lesion (i.e., tumor) (81). Prolonged psychosis and seizures in the setting of unresponsiveness, dyskinesias, autonomic instability, and hypoventilation, particularly in women, should prompt consideration of an anti–NMDA receptor antibody encephalitis.
The most common form of psychosis occurs between seizures (interictal) (82). Interictal psychosis is present in up to 9.4% of patients with MTS. Average age of onset is approximately 25 to 30 years (83,84), at a mean of nearly 15 years after the first unprovoked seizure (83). Earlier onset of psychosis is associated with generalized epilepsy and a positive family history of psychosis (83). Symptomatology appears to be similar across epilepsy syndromes (78). Features resemble that of schizophrenia, with persistent or recurrent positive symptoms, such as delusions and visual or auditory hallucinations, in the setting of otherwise clear consciousness (85,86). Themes are often persecutory or religious and may have strong affective components. Common associated affective changes include irritability, depression, and aggressive behavior. As in schizophrenia, symptoms may be insidious in onset. Episodes tend to be long-lasting, but may vary in duration, from minutes to years. The mean duration in a study of 155 patients was approximately 80 weeks, with half of the episodes lasting 4 months or longer. The duration tends to be shorter with advancing age (84).
Many key differences to schizophrenia, however, have prompted the term “schizophrenia-like psychosis” of epilepsy (86). Compared with the psychosis of schizophrenia, patients with interictal psychosis often have an absence of negative symptoms or formal thought disorder, better premorbid states, and less deterioration of personality (87), although more recent data suggest that most patients with epilepsy and psychosis will have at least one negative symptom (78). Patients with psychosis related to epilepsy also have a slightly older age of onset compared to those with schizophrenia, with symptoms beginning in the mid-20s to mid-30s (83,84,87,88). Those with epilepsy-related psychosis are more likely to be male, as opposed to patients with schizophrenia (87). Patients with interictal psychosis may also have a better prognosis, with a tendency for remissions and positive responses to treatment (87).
In some patients, a positive correlation exists between overall seizure frequency and psychotic symptoms. A notable exception to this pattern, however, is the concept of “forced normalization” or “alternative psychosis” introduced by Landolt in 1953 (85). Although evident with other psychiatric disorders as noted above, forced normalization is classically associated with psychotic behavior. The patient may have periods of psychosis coinciding with improved seizure control or reduced epileptiform discharges on EEG, often seen with the addition of a new AED. Such periods may alternate with epochs of improved psychiatric function in the setting of a paradoxical increase in seizure frequency or abnormalities on EEG. The underlying pathophysiology is unclear. As Nadkarni et al. (81) note, the psychosis may also be a reaction to the new drug, with improved EEG patterns representing an epiphenomenon. AEDs associated with psychosis include DPH, LEV, TPM, ZNS, tiagabine, ezogabine, perampanel, and vigabatrin (82,89–93). Although generally well tolerated, a case of lacosamide-induced psychosis was also recently reported (94). Forced normalization has been documented with other treatments as well, including VNS (95).
De novo psychosis after epilepsy surgery has also been reported, with rates varying from <1% to 28.5%. Symptoms most often occur transiently after surgery, and the diagnosis may be easily missed. The time period of greatest concern is the first 6 months after resection. Risk factors include a family history of psychosis and surgery after 30 years of age. Some authors suggest an increased incidence in those undergoing nondominant temporal resections, although this is not a consistent finding. Etiology of epilepsy does not appear to affect risk assessment (81). While preoperative psychosis is a risk factor for postoperative psychosis, it should not pose a contraindication for surgery, as long as the patient has appropriate psychiatric care, can cooperate with evaluation and treatment, and understands associated risks and benefits (31).
PIP is less common, occurring in 6.4% of patients with MTS (77). It typically presents after a cluster of seizures or status epilepticus, oftentimes in someone whose seizures were otherwise well controlled. PIP after a single seizure is rare. Symptoms often begin after 24 to 48 hours of normal baseline behavior, a period termed the “lucid interval.” Episodes may last a few days to several weeks, terminating within 1 to 2 weeks on average. Approximately 95% of episodes will resolve within 1 month. A history of interictal psychosis, a family history of psychosis, and low intellectual functioning predict a longer duration of symptoms (96). Symptoms may include visual or auditory hallucinations, paranoia, delusions, confusion, affective changes, violence (i.e., suicidal acts), and amnesia (76,97,98). Religious or grandiose themes among hallucinations and delusions are common, while thought insertion, commenting/command hallucinations, and negative symptoms are rare. Most religious conversions occur during this period.
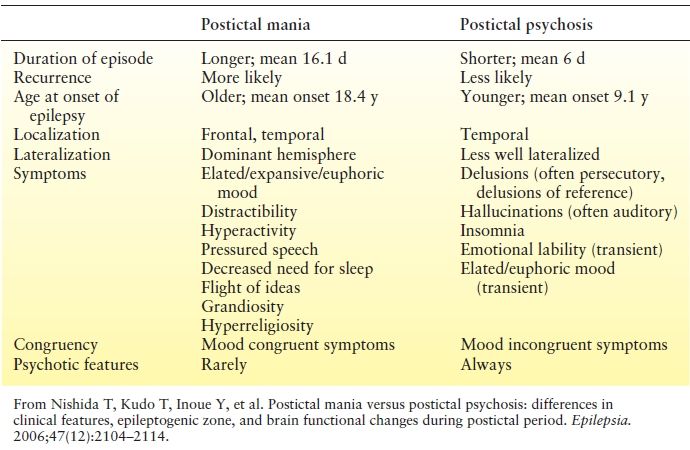
It is important to distinguish between PIP and postictal mania, as treatment options differ (Table 93.4). The two entities may be easily confused, as both may involve manic features and exhibit a similar lucid period. Logsdail and Toone (97) suggested the following diagnostic criteria for PIP:
Table 93.4 Comparison of Postictal Mania and Psychosis
1. An episode of psychosis developing within 1 week of a seizure or cluster of seizures
2. Lasting ≥24 hours and ≤3 months
3. Characterized by disorientation, delirium, delusions, or hallucinations in clear or clouded consciousness
4. Without AED toxicity, nonconvulsive status on EEG, prior interictal psychosis, recent head trauma, or alcohol/drug intoxication
Possible risk factors for PIP include age >30 years; male gender; focal-onset seizures; bilateral onset (often bitemporal) or spread of seizures (i.e., secondary generalization); a history of status epilepticus; prior encephalitis or other widespread CNS injury, borderline intelligence; EEG slowing; psychiatric illness; clusters of seizure activity; presence of ictal fear; and a family history of mood disorders, alcohol use, or epilepsy (98–101). Age at seizure onset and seizure frequency may not be predictive, although data are conflicting. These patients tend to have complex presentations, in that bitemporal dysfunction on neuropsychological testing may be greater than expected based upon structural imaging, and seizure onsets on video–EEG monitoring are often nonlateralizing (101). Postictal psychoses typically develop after at least 10 years of epilepsy and occur almost exclusively in adults, with the mean age of onset 32 to 35 years. Recurrent episodes have been documented in 12% to 50% of cases. As the frequency of psychotic episodes increases, the risk for developing chronic interictal psychosis becomes greater (81). In a series of 18 patients with PIP, 39% also experienced interictal psychosis (102).
Treatment
The first step in treatment is identification of the problem. Patients may not report their symptoms; hence, direct questioning is necessary. As “psychotic episodes may beget psychotic episodes,” once identified, symptoms should be treated immediately (81). On average, earlier antipsychotic administration significantly shortens episode duration (84).
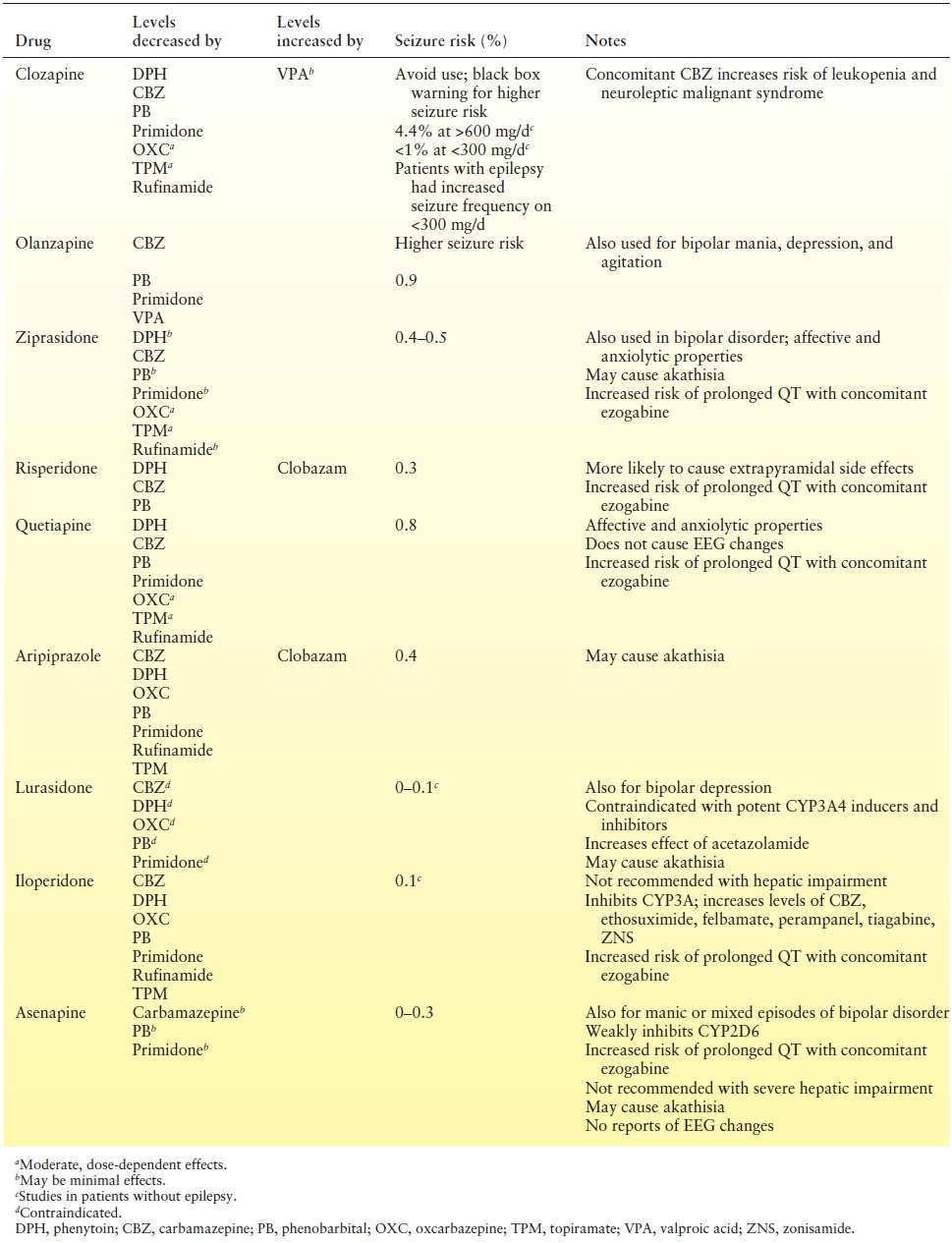
For those with peri-ictal psychosis, optimal seizure control is advised. Symptoms may resolve with treatment of the seizures (i.e., with resection) (79). Antipsychotic medications are the mainstay of management for both acute episodes and prevention, as long-term treatment may be necessary for patients with interictal or frequent peri-ictal episodes (Table 93.5). The ILAE guidelines make specific recommendations regarding the duration of treatment, suggesting a taper after 5 days for brief postictal psychotic episodes, a taper after 1 to 2 months when PIP lasts more than a few days, and long-term continuation for interictal psychosis (31). Some patients require psychotherapy, day treatment programs, case managers, or assisted living facilities, as well (81). ECT may be helpful in refractory cases. Patients with psychosis are best referred to epilepsy centers with teams that include psychiatrists and social workers.
Table 93.5 Atypical Antipsychotics
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








