13 Radiation Therapy Radiation therapy has an important role in the local control of sellar and parasellar tumors that are not completely resected, recur after surgery, or are considered at high risk for recurrence despite surgical resection based on anatomic and/or biological factors. This chapter outlines the various delivery systems and fractionation schemes used in radiation therapy, as well as the data supporting its use in both benign and malignant conditions. The term fractionation refers to the number of individual radiation treatments delivered. The total dose is the number of fractions multiplied by the dose per fraction. Clinical outcomes, such as local control, are heavily influenced by both the dose per fraction and total dose. For example, 50 Gy in 25 fractions may be equivalent in some tumors to 12 to 14 Gy in one fraction. Prolonging fractionation, by dividing the total dose into a larger number of fractions, has the potential to decrease late complications in normal tissue. This comes at the expense of a longer treatment time, which allows interfraction tumor repopulation and necessitates a higher total dose. The balance between these factors determines the relative benefits of conventional fractionated external beam radiation therapy (EBRT) versus those of stereotactic radiosurgery (SRS). The term conventional fractionation refers to the use of 1.8 to 2 Gy per day. As detailed in this chapter, the total doses for fractionated EBRT in the management of sellar/parasellar tumors range from 45 to 54 Gy, which implies 25 to 30 fractions, or 5 to 6 weeks of daily treatment. Treatment planning with three-dimensional conformal radiation therapy (CRT) is available in several commercially available software packages and typically requires at least three unopposed beams to reduce heterogeneity (“hot spots”) and minimize dose in surrounding uninvolved tissue. A growing number of radiation oncology practices use inverse-planned intensity-modulated radiation therapy (IMRT) for cases with a difficult geometric relationship between target and normal tissue, such as tumor surrounding the optic nerve in a concave configuration. There are several methodologies for IMRT, but most commonly require the capacity to divide the primary beam into 5- to 10-mm beamlets and the use of software to iterate and evaluate fluence through these beamlets to achieve the plan objectives. In all cases of linear accelerator (LINAC)–based intracranial radiation therapy, beam energy should be 6 to 10 megavolts (MV). In SRS, a high dose can be delivered in a single fraction by using a highly accurate and reproducible three-dimensional coordinate system for localization. Typical SRS doses for intracranial tumors range from 12 to 35 Gy, delivered in one dose. Several stereotactic systems exist to achieve submillimeter precision, the most common of which are the gamma knife (Elekta, Stockholm, Sweden), CyberKnife (Accuray, Sunnyvale, CA), and linear accelerator–based systems. For example, the gamma knife uses a metal collimator helmet for patient immobilization and distancing from the treatment head, which consists of a hemispheric distribution of sources of cobalt 60 behind bores. Bores are systematically removed such that beam arrays converge at an isocenter. Several overlapping isocenters may be used for irregularly shaped targets. LINAC-based systems achieve immobilization with (1) a rigid frame secured to the calvarium by four pins above the level of the brow, (2) a mask that incorporates a bite piece that relies on the uniqueness of the patient’s dental impression, or (3) a mask and image guidance system. The standard beam from a standard LINAC can be shaped by either cones or multileaf collimators (MLCs). A steep dose gradient between the target and adjacent normal tissues is created by (1) using multiple beam arrangements or arc therapy and (2) prescribing to the steepest portion of the beam profile, which is often the 50% isodose line (IDL) for gamma knife or the 80 to 90% IDL for LINAC-based SRS. SRS has advantages over fractionated EBRT: convenience, the ability to spare normal tissue, and minimal toxicity.1 For secretory pituitary adenomas, SRS may provide faster hormonal ablation.2 However, many patients are not candidates for SRS because of unfavorable tumor size, irregular geometry, or tumor location. Although each case is considered individually, the dosimetric advantages of SRS generally decline when the tumor is larger than 3 cm. For tumors abutting critical organs at risk (OARs), it is often difficult to achieve sufficient dose falloff between the prescription dose and the tolerance of the OAR. For example, to keep the optic chiasm and nerves from receiving less than 8 to 10 Gy at typical SRS doses, the target should generally be located at least 3 to 5 mm away from the optic apparatus. Historically, the 5-year risk for visual deficits from standard fractionated EBRT to the optic chiasm and nerves was believed to be 5% at 50 Gy and 50% at 65 Gy.3 However, these guidelines were based on consensus agreement rather than actual clinical data. In 2010, the Quantitative Analysis of Normal Tissue Effects in the Clinic (QUANTEC) provided updated recommendations for safe irradiation of the optic nerve and chiasm, suggesting that radiation-induced optic neuropathy (RION) is extremely rare at less than 55 Gy with fraction sizes of 1.8 to 2 Gy per day. The risk increases to 3 to 7% at doses of 55 to 60 Gy, and to more than 7 to 20% at doses above 60 Gy.4 Some series have suggested that tolerance may be lower and toxicity latency shorter in patients with pituitary tumors. Complications have been reported at doses as low as 46 Gy at 1.8 Gy per fraction, with average latencies of 10.5 and 31 months for pituitary and nonpituitary targets, respectively.5–7 When radiation is delivered as a single fraction, several series have shown that that rate of RION is very low at doses of less than 8 to 10 Gy, increases with doses from 10 to 12 Gy, and reaches more than 10% at 12 to 15 Gy.4,8 Therefore, the current recommendations for optic chiasm and nerve tolerance are below 55 Gy with standard fractionation and from below 8 to 10 Gy with SRS approaches. The brainstem tolerance is thought to be 12 Gy in a single fraction for 1 cm3 or more of tissue. Protons and other positively charged heavy particles hold an inherent advantage over photon therapy in their ability to deliver an equivalent dose to the target while minimizing exit the dose. When protons interact with normal tissues, their velocity rapidly decreases near their end of range. As this occurs, the energy transferred increases exponentially until the particles come to rest. The region of maximum energy transfer is called the Bragg peak. Superimposing beams of successively lower energies and intensities creates a spread-out Bragg peak, which delivers the intended dose to a three-dimensional target. The primary dosimetric advantage of proton therapy is the minimization of low and medium doses to nearby normal brain tissue. The clinical implication in the treatment of brain tumors is the theoretic reduction in risk for neurocognitive deficits and second malignancy, which are believed to be associated with even low doses of radiation. Both of these late sequelae are particularly important in the pediatric population. Confirmatory prospective studies are sparse, but such studies are currently under way at Massachusetts General Hospital. Proton therapy can be used for both stereotactic radiosurgery (PSRS) and fractionated stereotactic radiation therapy (PSRT) with noninvasive immobilization devices in a manner analogous to photon-based therapy. In the current era, the widespread use of proton therapy is hampered by the expense associated with such facilities. Radiation therapy plays a large role in the management of pituitary adenomas that have been incompletely addressed by surgical resection or have recurred biochemically or radiographically, and in the management of the rare medically inoperable patient. Extrasellar residual disease after resection and inadequate biochemical control with medications are common indications for radiation therapy. In the current era, SRS is preferable when the tumor geometry is favorable and the dose limitations to the optic apparatus and brainstem can be achieved. SRS is associated with faster biochemical normalization for secretory tumors.1,2,9 The technique and outcomes of stereotactic approaches are discussed in Chapter 14. For tumors with considerable extrasellar extension or in close proximity to the optic apparatus or other OARs, fractionated EBRT should be considered the standard of care to minimize the risk for late complications.9 The success of conventional fractionated EBRT in providing radiographic local control in nonfunctioning pituitary adenomas is ~95%. However, the actuarial rate of biochemical normalization of functioning tumors ranges from 50 to 100%, depending on histology.10–12 The first step in radiation planning is the definition of the target. The gross tumor volume (GTV) is the grossly visible pituitary adenoma by magnetic resonance imaging (MRI) and computed tomography (CT), which may extend outside the sella turcica into the cavernous sinus, sphenoid sinus, or intracranial parenchyma. The clinical target volume (CTV) encompasses the GTV as well as the extent of subclinical disease and typically includes the entire sella turcica and medial walls of the left and right cavernous sinuses. To create the planning target volume (PTV), the CTV is uniformly expanded by an additional margin to account for setup error and physiologic motion. The size of PTV expansion may range from 0 to 5 mm and depends on the reproducibility of the immobilization device used. Although there is 3 to 5 mm of setup uncertainty when a standard thermoplastic mask is used, when a stereotactic mask is used, the PTV expansion may be excluded altogether. OARs, such as the brainstem, optic apparatus, and temporal lobes, should also be defined. There are various dosimetric strategies available to achieve conformality to the target. Stereotactic radiosurgery (SRS) or stereotactic radiotherapy (SRT) techniques in which multiple non-coplanar arcs or pseudoarcs are used generally achieve the sharpest gradient between PTV and OARs. In centers without stereotactic capabilities, three-dimensional CRT with laterals, obliques, and/or a vertex field has been a standard approach for many years. Fractionated doses for pituitary adenomas range from 45 to 54 Gy, depending on tumor type. Radiation therapy is indicated for patients who have non-functioning adenomas (NFAs) that are unresectable or subtotally resected and who are felt to be at high risk for impending symptoms. The goal of radiation in this setting is to halt radiographic progression and prevent the development of symptoms. Prophylactic radiation after gross total resection or near-total resection is not indicated because the risk for recurrence is very low.13–16 If safe, repeat surgical debulking is often used as a strategy to delay radiotherapy (RT) and minimize the risk for hypopituitarism and other late sequelae.15,16 Both fractionated and SRS approaches are associated with excellent rates of local control (95–100%) but should be used judiciously because of the risk for late effects. Conventional fractionated EBRT at doses of 45 to 50.4 Gy in 1.8-Gy fractions has a long history of success in the treatment of NFAs. A retrospective experience of two hospitals in the United Kingdom, each with a different philosophy in management, included 126 patients with nonfunctioning pituitary adenomas.17 One hospital routinely offered postoperative RT to 45 Gy in 30 to 33 fractions to its 63 patients, whereas the other did not. The 15-year progression-free survival (PFS) significantly favored the subgroup of patients who received RT (93 vs. 33%, P < < 0.05). Similarly, a group in the Netherlands compared 76 patients who received immediate postoperative RT ranging from 45 to 55.8 Gy at 1.8 to 2 Gy per fraction with 28 patients who were followed with a wait-and-see policy.18 The 10-year local control rate was 95% for the RT group versus 22% for the wait-and-see group. Of note, there were no significant differences between the two groups with regard to need for hormonal supplementation or survival. SRS can be used to successfully treat NFAs when they are geometrically favorable (eg, <3 cm in size and >5–10 mm from the optic chiasm). A review of 62 patients who received gamma knife radiosurgery with a median marginal dose of 16 Gy and follow-up of 64 months reported 3- and 7-year local control rates of 95%.19 A prospective study that evaluated the efficacy of LINAC-based radiosurgery for non–surgically accessible adenomas included 37 patients with NFAs and reported 100% local control at a mean follow-up of 56.6 months and a dose of 13.4 Gy.20 If adequate sparing of normal tissue cannot be achieved with single-fraction SRS, fractionated SRT may be used because it combines the precise immobilization of SRS with the radiobiological benefits of standard fractionation on normal tissue. Colin et al reported a series of 63 patients with NFAs who were treated with fractionated SRT to 50.4 Gy at 1.8 Gy per fraction and followed for a median of 82 months; local control was 100%.21 No late complications were reported, with the exception of pituitary deficiency in which the probability of requiring hormonal replacement was 28.5% at 4 years and 35% at 8 years. In a limited number of centers, proton therapy is used to deliver treatment doses to gross disease while minimizing exit dose. In the Loma Linda experience, 24 patients with NFAs were treated to a median of 54 cobalt gray equivalents (CGE), with achievement of 100% local control at a median follow-up of 47 months.22 To achieve a relatively rapid biochemical remission, functioning adenomas typically require higher doses than NFAs. A dose response favoring the delivery of 45 Gy or more was reported in a retrospective review from Zierhut et al that included 139 patients with pituitary adenomas of various histologies;12 however, this is not a universal finding.23 The recommended fractionated EBRT dose is 45 to 54 Gy in 1.8- to 2-Gy fractions for acromegaly, Cushing disease, and prolactinoma. Because they are felt to be less radioresponsive, thyroid-stimulating hormone (TSH)–producing adenomas are often treated with a slightly higher dose (eg, 54 Gy). Preferences for SRS doses for functioning adenomas range widely, from 18 to 35 Gy in a single dose. The latency period between treatment and biochemical normalization can be several years, during which medical suppression is required. When feasible, SRS is favored because of the shorter latency period required to achieve hormonal normalization. For example, a comparison of SRS versus standard fractionated EBRT in 29 hormonally active adenomas reported a mean time to normalization of 8.5 versus 18 months, respectively.9 Growth Hormone–Secreting Adenomas RT is often used for patients with acromegaly who have suprasellar components that are unresectable or are incompletely controlled by surgery or medical therapy. Although radiographic local control may be quickly achieved with RT, biochemical and clinical normalization often requires many years. The most commonly accepted criteria for biochemical remission are normalization of insulin-like growth factor 1 (IGF-1; matched for age and gender) and a growth hormone (GH) level below 1 ng/mL after glucose challenge. There is wide variation in the reported biochemical remission rates for SRS and standard fractionated EBRT, but this likely reflects the long follow-up required to document maximized results, often 10 to 20 years. Standard fractionated EBRT has been in routine use much longer than SRS, and this allows the literature to demonstrate the long-term follow-up required to maximize biochemical remission rates. The largest retrospective experience included 884 patients at 14 centers in the United Kingdom.24 GH levels fell to below 2.5 ng/mL in 22% at 2 years, 60% at 10 years, and 77% at 20 years. Sixty-three percent achieved normal IGF-1 levels at 10 years. Similarly, Barrande et al reported a long-term single-institution experience of 128 patients with acromegaly treated with fractionated radiation.25 At a median follow-up of 11.5 years, basal levels of GH below 2.5 ng/mL were achieved in 7% at 2 years, 53% at 10 years, and 66% at 15 years. At last follow-up, 79% had achieved IGF-1 normalization. A smaller Dutch experience of 36 patients that used a median of 40 Gy reported IGF-1 normalization rates of 60%, 74%, and 84% at 5, 10, and 15 years, respectively, of follow-up.26 Radiographic local control is immediate and almost universally achieved.27,28 Biochemical remission rates in large series of SRS range from 40 to 96%, but this wide variation likely reflects differences in defining biochemical remission and insufficient follow-up. A large systematic review of pituitary adenomas treated with SRS included 420 patients with acromegaly in 25 retrospective studies.1 The mean marginal doses ranged from 15 to 34 Gy. Remission rates ranged from 0 to 100%, but the criteria were variable and often poorly defined. The use of somatostatin analogues during and after SRS often went unaccounted. The largest single series, published by Castinetti et al, included 82 patients treated with gamma knife to a marginal dose of 12 to 40 Gy.29 At a mean follow-up of 50 months, 17% achieved a biochemical remission, defined as a GH level below 2 ng/mL and normalized IGF-1 after discontinuation of somatostatin agonists for at least 3 months. An additional 23% regained control of GH secretion with medical therapy. A study by Zhang et al included 68 patients with acromegaly treated with gamma knife to a mean marginal dose of 31 Gy.30 Biochemical remission (generously defined as GH <12 ng/mL) was achieved in 40% at 12 months but reached 96% at 24 months. Kobayashi et al reviewed 67 patients treated with gamma knife to a mean marginal dose of 18.9 Gy.31 At a mean follow-up of 63 months, GH levels significantly decreased (<5 ng/mL) in 41%. Serum IGF-1 levels also significantly decreased (<400 ng/mL) in 41% of cases. Proton therapy is currently available in a selected few institutions for the treatment of acromegaly. The Massachusetts General Hospital experience included 22 patients treated with PSRS for refractory disease after transsphenoidal resection.32 After delivery of a median dose of 20 CGE and 6.3 years of follow-up, 59% achieved biochemical complete response (CR), defined as sustained IGF-1 normalization without medical therapy. In these patients, the median time to CR was 3.5 years, suggesting a more rapid response with SRS compared with standard fractionated EBRT. The Loma Linda experience reported by Ronson et al included 11 patients with GH-secreting adenomas treated with fractionated proton therapy to a median dose of 54 CGE and documented biochemical normalization or improvement in 45% and 36%, respectively, at a median follow-up of 3.9 years.22 Predictors of biochemical response are poorly defined, but there is a suggestion that lower baseline IGF-1 and GH levels are associated with higher rates of normalization.29,33,34 In SRS, there is some concern that the use of octreotide at the time of radiosurgery is associated with a longer latency period to biochemical response,35 although this is not a universal finding.29 ACTH-Secreting Adenomas In Cushing disease, RT is commonly used for patients with radiographic residual or recurrent disease after transsphenoidal surgery or in patients with no radiographic evidence of disease but with excessive hormone production. Biochemical remission is typically defined as normalization of urinary free cortisol (UFC) and serum adrenocorticotropic hormone (ACTH), but these criteria vary widely in the literature. Both standard fractionated EBRT and SRS appear to achieve biochemical remission rates of 50 to 80%. Estrada et al included 30 patients with Cushing disease treated with 48 to 54 Gy and reported actuarial remission rates of 44% at 1 year and 83% at 3 years.10 In 40 patients with Cushing disease treated with 45 to 100 Gy, Hughes et al reported an actuarial 10-year PFS rate of 59%.36 In the SRS literature, remission rates are 35 to 63% with shorter-term follow-up when compared with the standard fractionated EBRT literature. For example, Sheehan et al treated 43 patients who had refractory Cushing disease with gamma knife to a mean marginal dose of 20 Gy and achieved 63% biochemical normalization at a median follow-up of 44 months.37 Although there have been no prospective comparisons, LINAC-based SRS and gamma knife SRS are believed to offer comparable results.9,38 Proton therapy seems to achieve remission rates similar to those of photon-based therapy while sparing normal tissue of exit dose. The Massachusetts General Hospital PSRS experience of 33 patients with ACTH-producing adenomas reported a 52% complete radiographic and biochemical response in patients off medical therapy after a median dose of 20 CGE at a median follow-up of 62 months.39 In more than 50% of these patients, no tumor was present on MR at the time of the treatment. Older data from the Lawrence Berkeley Laboratory on the use of helium ions, another heavy charged particle, to treat 83 patients with Cushing disease with 30 to 150 Gy in three to four fractions demonstrated an 85% biochemical cure rate.40 Prolactinomas RT for prolactin-secreting adenomas is very uncommon because the combination of medical therapy (eg, dopamine agonists) and surgery achieves biochemical remission and radiographic stability in the vast majority of cases. In the event that either fractionated EBRT or SRS is required, their success without concurrent pharmacotherapy seems to be poorer than in other functional adenoma subtypes. Biochemical remission, often defined as normal serum prolactin of less than 20 to 25 ng/mL depending on sex, occurs in only 15 to 30% of cases with radiation alone and often requires a latency period of many years. For example, Littley et al reviewed the success of standard fractionated EBRT (20–42.5 Gy in eight to 15 fractions) in 58 patients and reported 71% prolactin normalization while they were on a dopamine agonist.41 When medication was discontinued, only 21% remained normoprolactinemic. In their comprehensive systematic review of SRS in pituitary adenomas, Sheehan et al included 393 prolactinomas across 22 series.1 The single-fraction dose ranged from 13.3 to 33 Gy. Remission rates varied from 0 to 84%, but the majority of studies reported 15 to 30% endocrine cure rates in those that clearly defined their end points. All but one SRS series included 21 or fewer patients. The largest series included 128 patients treated with gamma knife to a marginal dose of 9 to 35 Gy and a median follow-up of 2.8 years.42 Although radiographic control occurred in 98% of cases, only 13% of evaluable patients at 2 years had durable biochemical normalization off bromocriptine. As in other functional adenomas, retrospective data in prolactinomas suggest a poorer response to radiation when patients are concurrently using pharmacotherapy. In a small gamma knife series of 20 patients, all five patients who achieved a complete biochemical remission were not on a concurrent dopamine agonist. Conversely, none of the nine patients using pharmacotherapy achieved a completeremission.43 These data should be interpreted with caution, given the low patient numbers in each of the retrospective studies. Nonetheless, the current literature suggests that although RT alone is sufficient to maintain radiographic stability, it rarely achieves biochemical normalization for prolactinomas. Radiation may be thought of as an adjunctive therapy to be used in combination with dopamine agonists and/or surgical resection. Thyroid-Stimulating Hormone–Secreting Adenomas TSH-secreting adenomas account for fewer than 1% of all functioning pituitary adenomas but are considered more resistant to all types of therapy. Transsphenoidal resection remains the most appropriate initial strategy but results in cure in only one-third of patients. Therefore, most patients will require pharmacotherapy, such as octreotide or bromocriptine, before and/or after surgery to maximize biochemical normalization. The data on RT for TSH-secreting adenomas are sparse. Because of their aggressive natural history, some would advocate radiation therapy to higher dose (54 Gy) in all cases of TSH-secreting adenomas. Craniopharyngiomas are benign cystic epithelial tumors that arise from the pars intermedia (remnant of the Rathke pouch) located between the anterior and posterior components of the pituitary gland. The bimodal distribution peaks at 5 to 14 and at 50 to 75 years of age. The majority are retrochiasmatic and may present with symptoms of increased intracranial pressure from compression of the third ventricle. Those that are prechiasmatic may lead to homonymous hemianopsia from compression of the optic chiasm. Maximal safe resection is the first step in the management of craniopharyngiomas to confirm the diagnosis, rapidly relieve symptoms related to mass effect, and de-bulk tumor and cystic components to assist in radiation planning. Radiation alone should not be expected to reduce tumor/cyst size and symptoms. Historically, aggressive surgery was the preferred treatment strategy because when a gross total resection can be achieved, the 5-year PFS rate is 80 to 90%. However, aggressive surgery carries significant risk for cognitive decline, hypothalamic injury, optic nerve damage, diabetes insipidus, and other endocrinopathies. In the current era, most multidisciplinary teams favor maximal safe resection with postoperative RT (if residual disease remains), which offers similar PFS rates in comparison with gross total resection. Limited surgeries may include partial resections or cyst aspiration for relief of symptoms. Cognitive comparisons of patients undergoing either of the two approaches (extensive surgery vs. limited surgery + RT) performed at St. Jude Children’s Research Hospital suggest that extensive surgery is associated with greater decline in full-scale IQ (9.8- vs. 1.25-point drop, P < 0.063) and quality of life.44 After subtotal resection alone, the 5-year PFS is less than 50%.45,46 However, the addition of postoperative radiation improves 5-year PFS to 80 to 90%.44,47,48 The Royal Marsden Hospital experience included 173 patients with craniopharyngioma treated between 1950 and 1986 with fractionated EBRT alone or with surgery. In this series, four underwent gross total resection. Median follow-up was 12 years. The 10- and 20-year PFS rates were 83% and 79%, respectively.47 Overall survival (OS) rates were 77% and 66%, respectively. The standard radiation dose for craniopharyngiomas is 50.4 to 54 Gy in 1.8-Gy fractions delivered to the tumor and cystic volumes after decompression. A margin of 1 to 2 cm may be added to include areas at high risk for microscopic extension. Three-dimensional CRT or IMRT should be used to minimize dose to adjacent normal tissues, including the optic chiasm, optic nerves, and brainstem. SRS after limited surgery is another promising first-line or salvage option for those with favorable geometry. The University of Pittsburgh experience of 46 patients treated with gamma knife to a median marginal dose of 13 Gy (range, 9–20 Gy) demonstrated a 5-year PFS rate of 92% for the solid tumor component and 68% when the cystic components were included. Mean follow-up was 5.2 years.49 Acute clinical deterioration during or shortly after RT is uncommon, but it has been a well-documented phenomenon that is usually caused by rapid cyst reaccumulation. The Royal Marsden Hospital cohort included 26 patients (14%) whose condition acutely deteriorated 2 months before, during, or after RT. Cystic enlargement with or without hydrocephalus was the most common cause (62%); in an additional 23% of the cases, the cause was hydrocephalus without clear cystic enlargement.50 All patients who received surgical intervention (eg, aspiration, resection, or shunt) survived the immediate period, whereas six of the seven without surgical intervention died. Therefore, early recognition and surgical intervention are paramount for the patient’s success, leading some to advocate scheduled brain imaging at regular intervals during RT. In summary, maximal safe resection is the initial step in the management of craniopharyngiomas for the establishment of a diagnosis, relief of compressive symptoms, and improvement of postoperative radiation dosimetry. When gross total resection can be safely achieved for small lesions, no postoperative RT is required. In larger tumors, limited surgery followed by standard fractionated EBRT is recommended to minimize late toxicities while preserving high rates of local control. SRS should be reserved for small or recurrent tumors at an adequate distance from the optic apparatus. Refractory cyst reaccumulation is often managed by intermittent aspiration or intralesional sclerosis with bleomycin or various β emitters. In very young children, attempts are often made to use limited surgeries and intralesional therapies as temporizing measures to delay RT and its neurocognitive late effects. Meningiomas arise from arachnoid cap cells between the dura and pia mater. When managed in a multidisciplinary setting, benign (World Health Organization [WHO] grade 1) meningiomas have a 90 to 95% local control rate and rarely limit life expectancy. As such, the management of benign meningiomas is a delicate balance between achieving long-term cure and minimizing treatment-associated toxicity. Special considerations are made for parasellar meningiomas, including those of the cavernous sinus, medial sphenoid wing, tuberculum sellae, and intracanalicular optic nerve sheath because of their intimate association with the optic apparatus. Because surgical biopsy carries high risk, RT is considered the primary therapy in these locations. Such lesions are typically diagnosed on radiographic appearance alone without tissue confirmation. In general, RT is effective for benign meningiomas after subtotal resection. The University of California at San Francisco experience included 140 patients treated after subtotal resection with a median dose of 54 Gy.51 This included 117 benign and 23 malignant tumors. With a median follow-up of 40 months, the 5-year PFS rate was 89% for all patients and 98% for those treated after the introduction of CT- or MRI-assisted treatment planning. Several other retrospective series of subtotally resected meningiomas have also reported 5-year PFS rates of 84 to 92%.52–54 By comparison, SRT alone historically yields a 5-year PFS rate of 50 to 60%. SRS is another common treatment choice for small meningiomas that are located at a sufficient distance from the optic chiasm and nerves. Pollock et al retrospectively compared outcomes of benign meningiomas smaller than 3.5 cm treated with either surgical resection (n = 136) or SRS (n = 62) with a mean marginal dose of 17.7 Gy.55 At mean follow-up of 5.3 years, there was no significant difference in 3- and 7-year PFS rates between SRS and Simpson grade 1 resections. When compared with less complete resections, SRS was associated with improved PFS. Proton therapy is used in a selected few institutions to deliver prescription doses to skull-based meningiomas while minimizing low- and medium-dose scatter to uninvolved brain tissue. Similar to the results in photon series, local control generally ranges from 90 to 100%, although most series have a short-term follow-up of 3 years or less.56–58 Higher-grade meningiomas have a much higher risk for recurrence after RT. Milosevic et al reported the Princess Margaret Hospital experience, which included 17 patients with atypical histology and 42 with malignant meningiomas based on brain invasion or histopathology.59 A median dose of 50 Gy was administered to patients who previously had undergone gross total resection (29%), SRT (59%), or other (12%). Progression occurred in 66% after RT, of whom 92% died of meningioma. The 5-year cause-specific survival was only 34%. The standard fractionated dose for benign meningiomas is 50.4 to 54 Gy in 1.8-Gy fractions. Retrospective data suggest better 5-year PFS rates with doses above 50 to 52 Gy.51,59 Grades 2 and 3 meningiomas are often treated to 59.4 Gy. The radiation target conventionally includes (1) the dural tails and (2) a margin for microscopic extension along dural surfaces of 1 to 2 cm depending on aggressiveness of disease. For SRS, the recommended dose is 12 to 15 Gy for WHO grade 1 and more than 17 Gy when feasible for WHO grades 2 and 3 meningiomas. Radiation treatment planning is challenging for meningiomas of the parasellar region because the adjacent critical tissues (eg, optic chiasm and nerves) carry tolerances at or below the recommended radiation dose (see preceding section, “Dose Limitations of the Optic Chiasm and Nerves”). Therefore, optic nerve sheath meningiomas are not treated with SRS because a therapeutic dose (12 Gy × 1) would exceed optic chiasm and nerve tolerance (8–10 Gy). With fractionated EBRT, most radiation oncologists would advocate using 45 to 50.4 Gy for optic nerve sheath meningiomas to minimize the risk for radiation-induced optic neuropathy. Results with fractionated conformal radiation are quite impressive for tumor control and visual restoration.60 Cavernous sinus meningiomas are rarely completely resectable and difficult to biopsy. Diagnosis relies heavily on radiographic findings. Because radiation alone offers excellent long-term local control of 93 to 100%,61–65 attempts at resection should be avoided unless for palliative decompression in symptomatic patients. A large experience from the University of Heidelberg included 57 patients treated with standard fractionated SRT as primary treatment (51%), following surgery (18%), or at the time of recurrence (32%); the median dose was 57.6 Gy.63 With a median follow-up of 6.5 years, local control was 100% and 10-year survival was 95.5%. Selch et al reported a more modern series of 45 patients treated between 1997 and 2002 with CT- or MRI-assisted conformal RT to a median dose of 50.4 Gy.64 RT was used as definitive, adjuvant, or salvage therapy. The overall 3-year PFS was 97.4%. SRS is an option for cavernous sinus meningiomas that are considered geometrically favorable. Radiosurgery can also reverse cranial neuropathies more rapidly than fractionated schedules. SRS is associated with a 5-year PFS of 90 to 95%66–71 and symptomatic improvement in 25 to 50%.66,67,69,71 Lee et al reviewed 159 patients with cavernous sinus meningiomas treated with gamma knife SRS with or without prior subtotal resection.67 The median marginal dose was 13 Gy. The 5- and 10-year local control rates were both 93%. Neurologic status improved in 29%. Another gamma knife series included 115 patients treated with a mean marginal dose of 13 Gy and reported 5- and 10-year local control rates of 94 and 92%, respectively.66 Forty-three patients (46%) experienced neurologic improvement. A similar LINAC-based SRS series by Spiegelmann et al reviewed 42 patients treated to a mean marginal dose of 14 Gy. At a median follow-up of 3 years, the local control rate was 97.5%. Decreases in pretreatment neuropathies were noted in 29% of cranial nerve (CN) V, 22% of CN VI, and 13% of CN IV deficits. SRS for cavernous sinus meningiomas is associated with a low combined risk for complications ranging from 1 to 7%.67–71 Of these, the most common risks include trigeminal neuropathy/neuralgia, diplopia, visual field deficits, and other manifestations of optic neuropathy. Other parasellar or suprasellar meningiomas, such as those arising from the tuberculum sellae or intracanalicular optic nerve sheath, are very rare. The tuberculum sellae is an osseous protuberance at the anterosuperior margin of the sella turcica that is covered by a layer of dura stretching as a roof over the sella toward the posterior clinoid. Anterior to this, optic nerve sheath meningiomas arise in the dural covering of the optic nerve and comprise only 1 to 2% of all intracranial meningiomas. Of these, the vast majority are intraorbital (92%), whereas 8% arise intracanalicularly.72 The published literature for these rare entities is scant. Available data suggest that maximal safe resection is possible in experienced hands and may be considered for symptomatic patients (ie, visual deficits) in need of immediate decompression. However, because of the likelihood of gross or microscopic residual disease, local recurrence is common. Chicani and Miller reported visual outcomes in 18 patients with suprasellar meningiomas treated at the Johns Hopkins Hospital.73 Although there were mixed long-term results with respect to visual outcome, 39% developed radiographic recurrence at a mean time of 10.7 years. Over half of the recurrences occurred after gross total resection. Similarly, for optic nerve sheath meningiomas, a summary of the literature by Dutton reported an overall recurrence rate of 25% after surgery.72 The operative complication rate was ~30%. Fractionated radiation alone appears to provide excellent local control and preserve or improve vision in most cases. Turbin et al retrospectively reviewed 64 patients with primary optic nerve sheath meningiomas who were treated with surgery alone, surgery and RT, or RT alone to 40 to 55 Gy.74 Mean follow-up was 12.5 years. There were no significant differences in visual acuity at baseline among the groups. After therapy, visual acuity significantly worsened in the surgery and surgery + RT groups but was unchanged in the RT-alone group. Complication rates were 62 to 68% in the surgical groups, but only 33% in the RT-alone group. Andrews et al reviewed 33 optic nerve sheath meningiomas treated with fractionated SRT to a median dose of 51 Gy with a median follow-up of 7.4 years.75 Of 22 optic nerves with pretreatment function, vision was improved in 42% and at least preserved in 92%. Importantly, there were no local recurrences. In contrast to SRS for cavernous sinus meningiomas, SRS for meningiomas of the tuberculum sellae or optic nerve sheath is not favored because the dose required for adequate local control (12–13 Gy) exceeds the optic chiasm and nerve tolerances (8–10 Gy). Therefore, high-precision fractionated SRT to 50.4 to 54 Gy should be considered the standard first-line therapy, given its favorable safety profile and success in local control. Taken together, among patients with suprasellar lesions, very conservative resection may be considered for those who might gain visual benefit from immediate decompression. For those with optic nerve sheath meningiomas, any attempt at resection or even biopsy is associated with an enormous risk for immediate blindness of the affected eye. Postoperatively, there should be a low threshold for RT because of the likelihood of residual disease. For all others (including those who are asymptomatic or have preexisting severe/complete visual loss), high-precision fractionated SRT is emerging as the treatment of choice because of its favorable toxicity profile and excellent local control. Historically, optic pathway gliomas (OPGs) have been considered a unique entity from other gliomas because of their poor surgical accessibility, predilection for patients of a young age, and association with neurofibromatosis type 1 (NF1).76 OPGs may occur in either the optic chiasm or nerves but often extend in an infiltrative pattern to the hypothalamus. Histology is typically a low-grade astrocytic tumor. They occur primarily in children, with 90% of cases occurring in patients younger than 20 years of age, and account for 5% of all pediatric central nervous system (CNS) tumors.77 There is an association with the NF1 gene; between 25 and 40% of childhood optic pathway tumors occur in children with NF1.78 The presenting symptom is most commonly visual loss, although children younger than 3 years of age are commonly brought to medical attention with proptosis, strabismus, nystagmus, or loss of developmental milestones.78 The overall management of OPGs draws from the principles used for other low-grade gliomas. Maximal safe resection should be considered initially to establish a diagnosis and provide immediate symptomatic relief if needed. Immediate postoperative radiation is often offered for those with symptomatic gross residual disease or at high risk for rapid progression. The timing of radiation should be determined on a patient-specific basis. A randomized trial of radiation given immediately after surgery versus at the time of progression found no difference in OS. However, immediate postoperative RT improved time to progression (4.8 years for adjuvant RT vs. 3.4 years for salvage RT).79 Radiation doses typically range from 50.4 to 54 Gy in 1.8 Gy per fraction.78 In adults with low-grade gliomas, randomized trials of 45 versus 59.4 Gy and of 50.4 versus 64.8 Gy have failed to demonstrate an improvement in 5-year OS with dose escalation.80,81 Age at diagnosis plays a crucial role in initial treatment decisions because RT in very young children is associated with neurocognitive late effects. Chemotherapy or close observation is often used to delay the need for RT to allow further brain maturation and obviate the side effects of treatment.82 The standard first-line chemotherapy is the “Packer regimen” consisting of carboplatin and vincristine (CV).83,84 Median delays in progression of 2.5 to 3 years can be achieved with chemotherapy, with 5-year PFS and OS rates of 56% and 90%, respectively.85,86 A completed but unpublished phase III Children’s Oncology Group trial (COG A9952) randomized patients between CV and TPCV (thioguanine, procarbazine, CCNU, and vincristine) in children younger than 10 years old with progressive or incompletely resected low-grade gliomas. Preliminary analysis suggests an overall response rate of ~60% with a 5-year event-free survival (EFS) of 35% for CV versus 48% for TPCV (P = 0.11).83,87,88 Although the success of chemotherapy at delaying radiation is encouraging, it must be recognized that the majority of patients will have progression within 5 years and require radiation by that time. OPGs are historically associated with a higher risk for progression yet retain a very favorable OS rate.89–91 This finding is likely related to the young patient demographics and the use of chemotherapy to delay RT, rather than intrinsic tumor behavior. The association between NF1 and OPGs is well established.92 OPG arising in the setting of NF1 is believed to have a more favorable natural history, and cases of spontaneous regression have been documented.93–95 Study A9952 has preliminarily reported a 5-year OS of 98% in patients with NF, which compares favorably with 86% in the non-NF1 population (P = 0.0017).87 However, radiation-related complications appear to be more common in the setting of NF1. In a series evaluating 58 patients with NF1 treated for optic gliomas, second primary tumors occurred in 9 of 18 (50%) following RT and in 8 of 40 (20%) without RT at a median of 12 years.96 Moyamoya syndrome is characterized by the appearance of abnormal collateral vascular networks adjacent to spontaneously occluded vessels of the circle of Willis. Patients with NF1 are more likely to develop moyamoya syndrome, and at a lower radiation dose threshold.97,98 These observations have led to a more conservative approach to the management of OPGs in NF1. Patients with asymptomatic OPGs are not treated—they are simply observed. Symptomatic patients receive chemotherapy as first-line therapy, with radiation reserved for truly refractory cases. In the current era, advanced technologies have improved the ability to spare normal tissue, which is of particular importance in the pediatric population. These include three-dimensional treatment planning, stereotactic immobilization to reduce the margin required for setup uncertainty, and proton therapy to minimize exit dose. A prospective trial evaluating the use of SRT with only a 2-mm margin for low-grade gliomas achieved 8-year PFS of 65% and OS of 82%, with no marginal failures.99 Long-term outcomes after RT are very favorable in children with OPGs, with 10-year survival rates of 80 to 90% (Table 13.1). Because of the risks associated with RT, it may be reserved until the time of symptomatic or radiographic progression, and this timing is heavily based on the patient’s age. Chemotherapy is often used as a strategy to delay RT and its potential neurocognitive effects. The primary role of surgery is for palliation of compressive symptoms and should be considered only on a patient-specific basis. Germ cell tumors are classified by the WHO system as pure germinomas (60% of intracranial germ cell neoplasms) and nongerminomatous germ cell tumors (NGGCTs, 40%). NGGCTs include teratomas, embryonal carcinomas, endodermal sinus tumors, choriocarcinomas, and mixed germ cell tumors.100 The primary lesion is located between the suprasellar cistern and pineal gland in 95% of patients with intracranial germ cell tumors, with the majority of germinomas found in the suprasellar region and of nongerminomatous tumors in the pineal area.100 Multiple midline germinomas are those that present with simultaneous involvement along the third ventricle, pineal, and suprasellar regions.101,102 At presentation, a triad of diabetes insipidus, visual field abnormalities, and anterior hypopituitarism may be observed. Other common symptoms are related to increased intracranial pressure, including headache, nausea, vomiting, and lethargy.103 The differentiation between pure germinomas and NG-GCTs is critical because this dramatically impacts prognosis and treatment decisions (Table 13.2). Complete staging should include MRI of the brain and total spine with contrast, measurement of tumor markers including serum and cerebrospinal fluid (CSF) β-human chorionic gonadotropin (β-HCG) and α-fetoprotein (AFP), and CSF cytology. Pure germinomas often present with markers within normal limits; however, some may have β-HCG levels above 100 IU/L. Definitive diagnosis by surgical biopsy is recommended in most cases. However, if surgery is considered high-risk, then any patient with elevated AFP should be assumed to have NGGCT as opposed to pure germinoma. RT has long been the standard treatment for pure germinomas and is an important component of multimodality therapy for NGGCTs.78,104 Pure germinomas are extremely radioresponsive. When RT alone is used for localized disease, 10-year OS exceeds 90%. However, late effects of RT can impact the neuropsychological function and quality of life of these patients.105 The historical treatment for pure germinomas has been craniospinal irradiation (CSI) to 36 Gy followed by a boost to the primary tumor to 50 to 54 Gy. However, the focus of treatment in recent years has shifted to decreasing the RT dose and volume while preserving curability. Debate continues over the most appropriate radiation volume. Choices include the local tumor only with a margin, tumor and third ventricle, whole ventricle, whole brain (WBRT), and full CSI. In a large meta-analysis of 754 patients, Rogers et al found that recurrence rates increased with smaller RT volumes: 4% following CSI, 8% following WBRT or whole-ventricle RT plus boost, and 23% following focal treatment alone.106 Importantly, the frequency of spinal relapse did not significantly differ between the CSI group and the whole-brain/whole-ventricle group (1.2% vs. 2.9%, respectively) but was significantly higher in the focal radiation group (11.3%). MAKEI 83/86/89 was a prospective dose reduction study of intracranial germinoma. In this study, CSI to 30 Gy with a 15-Gy boost was compared with CSI to 36 Gy with a 14-Gy boost (all in 1.5-Gy fractions). There were no statistically significant differences in outcomes.107 Complete remission was achieved in all 60 patients, with a 5-year relapse-free survival of 91% at a mean follow-up of 59.5 months. Currently, most advocate for treatment with whole-ventricle radiation doses of 21 to 24 Gy, with an additional boost to the primary tumor to 40 to 45 Gy. For patients with evidence of CSF dissemination at diagnosis, standard treatment remains CSI followed by a boost to the primary tumor and macroscopic metastases.106,108,109 Germinomas are highly responsive to chemotherapy, and this has led to combined-modality therapy trials attempting to further reduce the role of RT in low-risk subgroups. ACNS 0232 was a phase III trial that randomized children to RT alone versus chemotherapy followed by response-based RT. Patients with localized disease randomized to radiation alone received 21 Gy to the whole ventricle followed by a boost of 24 Gy to the primary site. Patients randomized to chemotherapy plus radiation received two to four cycles of chemotherapy (carboplatin, etoposide, cisplatin, and cyclophosphamide). If a complete response was achieved after two or four cycles, patients received 30 Gy to the involved field only. Unfortunately, the trial accrued poorly and closed early. Further studies are needed to determine whether the inclusion of chemotherapy will reduce the need for RT. NGGCTs are much rarer than germinomas, and therefore their management is guided by far fewer available data. Outcomes of NGGCTs are worse than those for germinomas, and optimal radiation dose and volume remain controversial. The current standard of care requires multimodality therapy consisting of platinum-based chemotherapy, CSI, and consideration of second-look surgery for resection of gross residual disease. Series evaluating the use of RT alone (CSI plus boost) in children with NGGCTs reported poor rates of OS of 20 to 40%.100,110,111 The use of neoadjuvant chemotherapy before RT has led to significant improvements, with survival rates of 60 to 70%.112,113 In POG 9530, 14 patients who had NGGCTs or who had germinomas with elevated AFP or β-HCG received four cycles of cisplatin and etoposide alternating with vincristine and cyclophosphamide.114 Five patients had a CR and received CSI to 30.6 Gy with boost to 50.4 Gy for local disease; nine patients had less than a CR and received CSI to 36 Gy with boost to 54 Gy. Probability of EFS was 79% at 58-month median follow-up. In the recently completed but unreported ACNS 0122, patients received carboplatin, VP-16, and ifosfamide followed by 36 Gy of CSI and an involved-field boost for a total dose of 54 Gy to the tumor bed. Second-look surgery was performed in those patients who did not achieve CR or partial response (PR) after neoadjuvant chemotherapy, and autologous peripheral blood stem cell transplantation was performed for persistently positive markers or evidence of residual malignant elements. Mature teratomas are a distinct entity from other NG-GCTs because they are theoretically benign and have a favorable prognosis. Most can be cured by complete resection.103 When this cannot be achieved, treatment with adjuvant radiation to a dose of 50 Gy has shown survival of up to 93% at 10 years.115 RT plays an important role in the management of both pure germinomas and NGGCTs. Emerging technology in radiation oncology may be able to further spare normal brain tissue by using IMRT or proton radiotherapy while preserving oncologic outcomes.116 Chordomas and chondrosarcomas arising near the skull base are managed fairly similarly, although they originate from different cell types and have different prognoses. Chordomas are rare, slow-growing, locally aggressive tumors, thought to originate from notochord remnants within the axial skeleton. Approximately 35% arise within the skull base, 50% in the sacrococcygeal region, and 15% in the vertebral column.117 Their metastatic potential is low, with reports ranging from ~5 to 20%. Failures are predominantly local in series with long-term follow-up.118,119 Chondrosarcomas are malignant tumors of cartilage-forming cells and can arise in any bone preformed by cartilage. They most commonly arise in the humerus, femur, or bones of the pelvis but can originate in the skull base and represent ~5% of skull base tumors.120 Skull-based chondrosarcomas are typically low-grade and progress slowly with relatively asymptomatic growth. This often leads to extensive locoregional infiltration by the time of diagnosis. For both chordomas and chondrosarcomas, radical resection by an experienced surgeon is the single most important aspect of treatment because local progression rather than distant metastasis is the main contributor to morbidity and mortality. Gross total resection can be difficult to achieve because of the anatomic constraints to surgical access, as well as the proximity of adjacent critical normal tissues. The volume of residual disease is a strong predictor of local control. In a series by Berson et al, patients with low-volume disease (<20 cm3) had a significantly higher local control rate after radiation than patients with larger-volume disease (80% vs. 33% at 5 years).121 Similar findings were reported by Hug et al.122 In patients with residual disease, postoperative radiation therapy is a vital component of care. In an early series of 155 patients with chordoma, there was a 1.5-year mean survival for patients who underwent surgery alone, compared with 5.2 years for those who underwent surgery followed by RT.123 Even with the addition of adjuvant RT, the pattern of failure is predominantly local.124 Similar to surgery, RT should be consolidated at experienced centers because of the apparent benefit of high doses. Radiation targets are defined as (1) areas of gross disease on imaging, (2) areas occupied by tumor preoperatively, and (3) anatomic compartments at risk for harboring microscopic disease. The total dose appears to the most important determinant of local control after radiation therapy. Rates of local recurrence after doses below 60 Gy have been as high as 70 to 100%, with most patients dying of locally progressive disease.119,125,126 In a more modern series, the use of advanced imaging and planning with doses of 50 to 64 Gy yielded 5-year PFS of only 23% and OS of 35%.127 However, dose escalation is often limited by the proximity of critical normal tissues, such as the brainstem and cranial nerves. Hug et al reported decreased rates of 5-year local control for tumors with brainstem abutment compared with those located further away (53 vs. 94%, respectively).122 When purposeful dose compromises are made at the tumor–critical structure interface, these “cold spots” within the tumor appear to be associated with the probability of recurrence.128 Also, for unclear reasons, female patients have higher local failure rates than their male counterparts. At the present time, there are no reported prospective randomized trials evaluating the value of dose escalation in these tumors. A randomized trial (NCT00592748) stratifying patients into low- versus high-risk groups, with doses of 69.6 Gy (relative biological effectiveness [RBE]) or 75.6 Gy (RBE) in the low-risk group and with doses of 75.6 Gy (RBE) or 82.9 Gy (RBE) in the high risk group, is closed to accrual and not currently reported. The use of charged particles, such as proton, helium, or carbon ion therapy, is strongly encouraged because of the ability to deliver high radiation doses to the tumor volume with minimal exit dose to critical structures. This strategy appears to improve local control compared with historical rates from photon therapy (Table 13.3). The treatment of macroscopic residual disease with conventional photon RT has yielded local control rates as poor as 27%.125 In contrast, the Massachusetts General Hospital experience included 290 patients treated with proton with or without photon therapy to doses of up to 83 CGE.129 At a median follow-up of 41 months, the 5- and 10-year local recurrence-free survival rates were 73% and 54%, respectively. The OS rates were 80% and 54%, respectively. Results from other experiences with charged particles have yielded similar results (Table 13.3). Series with modern photon techniques for dose escalation, such as SRT and three-dimensional CRT, suggest improved results for these in comparison with conventionally delivered photons. For example, Debus et al used stereotactic fractionated photons at a median dose of 66.6 Gy and reported 5-year local control rates of 50% for chordomas and 100% for chondrosarcomas.130 Late effects of RT are important to consider, given the long life expectancies that may be achievable with high-dose radiation therapy after safe maximal resection. Late effects were reported in 8% of patients in a proton series at Massachusetts General Hospital, which included asymptomatic or symptomatic brain changes, unilateral or bilateral blindness, and unilateral deafness.124 Temporal lobe damage has been reported in 7.6% of patients at 2 years and 13.2% at 5 years.131 Pituitary carcinomas are rare entities that comprise ~0.2% of pituitary tumors.132 They are defined as pituitary tumors with metastasis outside the CNS or occurring as separate foci within the CNS. Histologic confirmation often reveals increased mitotic activity (6 per 10 hpf) and p53 overexpression.133 Retrospective series suggest that ~75% are hormone-secreting and the remaining 25% non-functioning.134 In a series of 15 patients, the mean latency period to metastasis was ~7 years, with greater tendency toward systemic metastasis than toward isolated craniospinal metastasis.134 Given their rarity, there is no consensus for treatment strategy. Outcomes are poor even after multimodality treatment, including surgery, radiation, and chemotherapy. Experience with fractionated involved-field radiation therapy (45–56 Gy) has been limited to case reports.132,135 In a case report, a long-term survivor of pituitary carcinoma was initially treated with adjuvant RT to the sella to 56 Gy and at the time of recurrence 8 years later received WBRT to 24 Gy.135 Large autopsy series report that 1 to 3% of patients with malignant tumors have pituitary metastases.136–138 They are seen most commonly with breast cancer and lung cancer primaries but can originate from a wide variety of primary sites.139,140 Neurohypophysial metastases are more common, although breast cancers appear to preferentially metastasize to the adenohypophysis. Presenting symptoms most commonly include diabetes insipidus at rates between 29 and 71%.138,139 Other symptoms may include anterior hypopituitarism, visual field defects, retro-orbital pain, and ophthalmoplegia.141 In a series of 36 patients with pituitary metastases, such symptoms were the first sign of disease in 56%.139 In this series from Morita et al, treatment was primarily surgical, and adjuvant RT to a median dose of 36 Gy was given to half of the patients. Median survival was ~6 months. Completeness of resection and radiation dose did not appear to be associated with OS. Kano et al reported an experience in which 18 patients were treated with gamma knife SRS to a median marginal dose of 13 Gy. The median survival was 5.2 months.142 Following SRS, 50% of patients experienced relief of neurologic symptoms, and the condition of 43% of patients with preexisting diabetes insipidus was felt to have improved. Decisions regarding choice of RT are largely driven by dosimetric considerations. Because of their close proximity to the optic chiasm, pituitary metastases are usually not amenable to SRS because therapeutic doses exceed chiasm tolerance. In the setting of multiple brain metastases, WBRT is recommended. If the sellar metastasis is solitary in nature or refractory to WBRT, then SRS, hypofractionated SRT, or involved-field RT may be considered as long as optic chiasm and nerve tolerances are respected. The risk for late effects after radiation is always related to the adjacent normal tissue that receives a clinically relevant dose. In the sella and parasellar region, these “organs at risk” include the pituitary gland, optic chiasm, optic nerves, brain parenchyma, and contents of the cavernous sinus—namely, cranial nerves and carotid vessels. In the modern era, the sole purpose of advanced technology in radiation planning is to minimize dose to normal tissues and spare the patient both acute and late effects. These technologies include CT-based three-dimensional planning, IMRT, stereotactic approaches to minimize setup uncertainty, and proton therapy to minimize exit dose. Most of the available data below are drawn from the large experience in treating pituitary adenomas with standard fractionated EBRT and SRS. Hypopituitarism is a common late effect of RT when the prescribed dose to the sella is in the 45- to 54-Gy range used to treat most benign neoplasms. Because the sella is often the target, the risk for hypopituitarism is impossible to minimize. The risk for new hypopituitarism affecting at least one axis is 20 to 60% at 5 years for both standard fractionated EBRT and SRS.10,11,37,143 For example, Estrada et al reported a 57% risk for new GH deficiency after treatment for Cushing disease.10 With longer follow-up, Minniti et al reported new hypopituitarism in 85% at 15 years.11 Hoybye et al reported 100% GH deficiency and 69% thyroid hormone deficiency with mean follow-up of 17 years after SRS for ACTH-secreting adenomas. More modern dosimetric analyses have attempted to identify predictors for hypopituitarism, such as dose parameters for the entire pituitary,144 infundibulum,145 and hypothalamus.146 At the recommended tolerance doses of less than 54 Gy in 1.8- to 2-Gy fractions and less than 8 to 10 Gy in a single SRS fraction, the risk for visual deficits is minimal. This is reviewed in the earlier section, “Dose Limitations of the Optic Chiasm and Nerves.” Clinical data come from large series, including a compilation of 11 series involving 1388 patients treated with standard fractionated EBRT. The risk for visual injury was 1.7%.147 Similarly, the Royal Marsden Hospital experience included 411 patients with pituitary adenomas treated with 45 to 50 Gy in 25 to 30 fractions and found a 1.5% incidence of visual deterioration at 20 years.148 The largest SRS experience for adenomas included more than 1600 patients over 35 series and reported a 1% risk for visual changes.1 As in most disease sites, the risk for second malignancy after RT is difficult to measure because it is heavily dependent on dose, treatment volume, length of follow-up, and underlying host genetics. Radiation-induced tumors are most commonly meningiomas, gliomas, and sarcomas. Based on data from the literature for pituitary adenomas, the long-term risk for a second malignancy after standard fractionated EBRT is 1 to 3% at 20 years.147–149 However, in a large review of SRS series including 1621 patients, there were no reported radiation-induced maligancies.1 In addition to SRS, other modern technologies such as three-dimensional CRT and proton therapy provide a theoretic reduction in risk for second malignancy by reducing the volume of normal tissue exposed to radiation. Radionecrosis within uninvolved brain parenchyma, such as the temporal lobes, is extremely uncommon when 45 to 54 Gy in 1.8- to 2-Gy fractions or standard SRS doses are delivered to the sella or parasellar region. The reported risk is less than 1%.1,147 Radiation dose to the carotid vessels and circle of Willis has been associated with a small risk for subsequent vascular events. Brada et al reviewed 331 patients treated at the Royal Marsden Hospital between 1962 and 1986 for pituitary adenomas with RT.150 The actuarial incidence rates for cerebrovascular accident were 4%, 11%, and 21% at 5, 10, and 20 years, respectively, from the date of RT. Compared with the general population in the United Kingdom, this was estimated to represent a relative risk increase of 4.1. However, this study did not include patient-related factors in the analysis, nor did it necessarily reflect modern radiation technologies and strategies. RT plays an important role in the management of benign and malignant tumors of the sellar and parasellar regions. In all of the neoplasms discussed in this chapter, there is a delicate balance between the benefits of local control and the risks for late complications of RT. Technology in radiation oncology is continuously evolving to improve the delivery of therapeutic doses to involved regions while minimizing dose to normal tissues. Most importantly, the optimal timing of RT is often difficult to ascertain and should be determined in a multidisciplinary setting.
 Fractionation Schedules and Technology in Radiation Oncology
Fractionation Schedules and Technology in Radiation Oncology
 Conventional Fractionated EBRT
Conventional Fractionated EBRT
 Stereotactic Radiosurgery
Stereotactic Radiosurgery
 Indications for SRS versus Conventional Fractionated EBRT
Indications for SRS versus Conventional Fractionated EBRT
 Dose Limitations of the Optic Chiasm and Nerves
Dose Limitations of the Optic Chiasm and Nerves
 Proton Therapy
Proton Therapy
 Benign Tumors
Benign Tumors
Pituitary Adenomas
Nonfunctioning Adenomas
Functioning Adenomas
Craniopharyngiomas
Meningiomas
Meningiomas of the Cavernous Sinus and Medial Sphenoid Wing
Meningiomas of the Tuberculum Sellae or Optic Nerve Sheath
 Malignant Tumors
Malignant Tumors
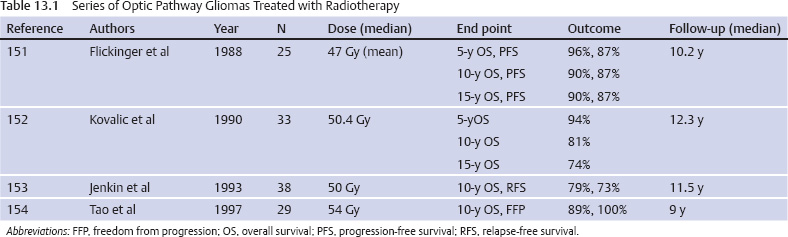
Optic Pathway Gliomas
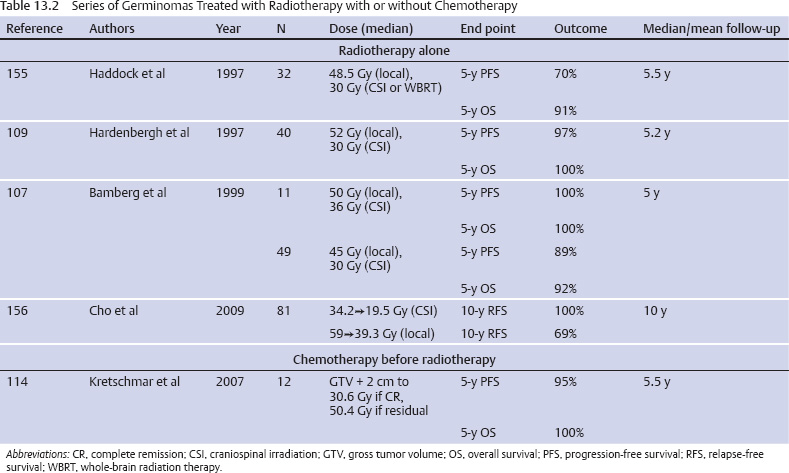
Germ Cell Tumors
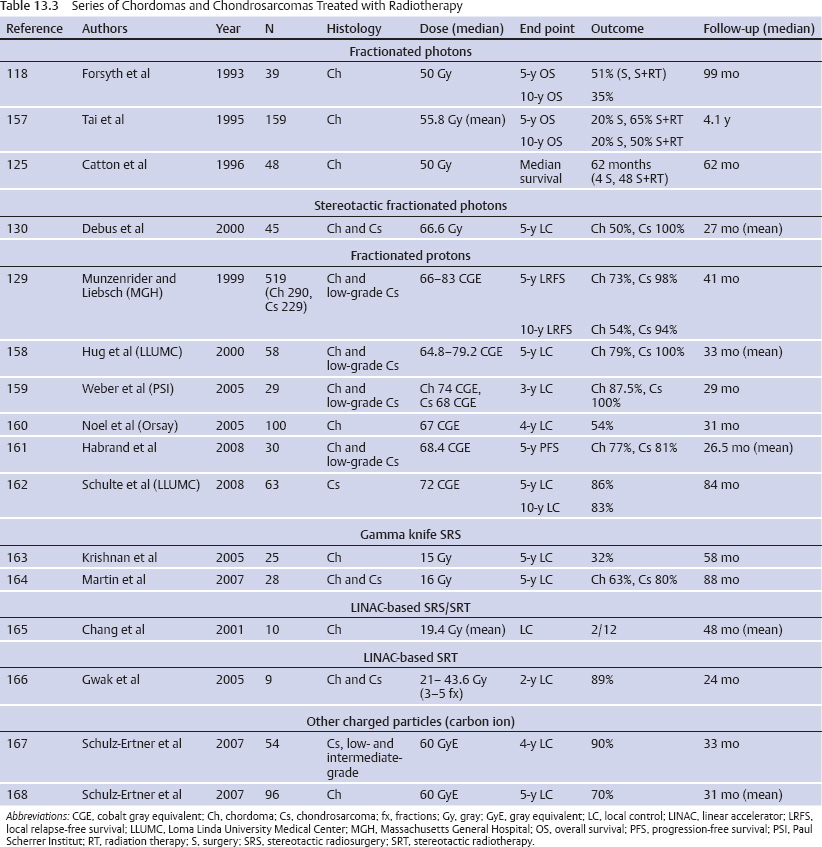
Chordomas and Chondrosarcomas
Pituitary Carcinomas
Sellar Metastases
 Late Effects of Radiation Therapy
Late Effects of Radiation Therapy
Hypopituitarism
Visual Deficits
Second Malignancy
Radionecrosis
Cerebrovascular Events
 Conclusion
Conclusion
References
 Orsay. Strahlenther Onkol 2002;178(9):480–485
Orsay. Strahlenther Onkol 2002;178(9):480–485