CLINICAL RESTLESS LEGS SYNDROME
Restless legs syndrome (RLS) is clinically defined by the presence of four criteria: (1) an urge to move the limbs with or without sensations, (2) worsening at rest, (3) improvement with activity, and (4) worsening in the evening or night (1). Additional criteria now stipulate that these symptoms not be caused by another etiology. The diagnosis of RLS is exclusively based on those symptoms. Diagnostic phone interview (2), rating scale (3), and quality-of-life scale (4) have all been validated based on these features.
Patient subjective descriptions, however, are quite varied and tend to be suggestible and education-dependent. The sensation is always unpleasant but not necessarily “painful.” It is usually deep within the legs and commonly between the knee and ankle. In one study of RLS patients, the most common terms used include “need to move,” “crawling,” “tingling,” “restless,” “cramping,” “creeping,” “pulling,” “painful,” “electric,” “tension,” “discomfort,” and “itching” (5). Patients usually deny any “burning” or “pins and needles” sensations, commonly experienced in neuropathies or nerve entrapments, although neuropathic pain and RLS can coexist. The key is to rephrase their description into a question asking if that “makes you want to move your legs,” is it better while moving, and are the symptoms worse in the evening/night.
Essentially, all patients report transient symptomatic improvement from walking, although other movements also help. Other therapeutic strategies reported by our patients include rubbing or pressure, stretching, and hot water. In general, harsh sensory stimuli can mitigate RLS. Cognitive or emotional activating circumstances (i.e., arguing) also often reduce RLS. Other clinical features typical for RLS include the tendency for symptoms to gradually worsen with age, improvement with dopaminergic treatments, a positive family history, and periodic limb movements while asleep. Traditionally, the neurologic examination is normal in RLS; however, leg/feet stereotypies (Video 34.1) are often present, though usually suppressible.
PERIODIC LIMB MOVEMENTS OF SLEEP
Periodic limb movements of sleep (PLMS) are defined by the Association of Sleep Disorders as “periodic episodes of repetitive and highly stereotyped limb movements that occur during sleep.” The incidence in the general population increases with age and is reported to occur in as many as 57% of elderly people (6,7). Therefore, PLMS are common in the general population as an age-dependent phenomenon.
PLMS are seen in more than 80% of RLS patients, depending on how many nights are evaluated, with increased percentages if more nights are investigated (8). Although PLMS accompany most cases of RLS, RLS prevalence in the setting of polysomnographically documented PLMS found that only 9 of 53 (17.0%) PLMS patients complained of RLS symptoms (9). Therefore, most people with RLS have PLMS but many patients with isolated PLMS do not have RLS. They are not part of the diagnostic criteria for RLS, and a polysomnogram (PSG) is not needed to diagnose RLS. Conceptually, some people segregate RLS into a sensory component (urge) and the motor component (PLMS) or periodic limb movements while awake; however, the relationship between the two is not clear (10). At least one gene is associated with both conditions (11).
PLMS can occur simultaneously in both legs, alternate between legs, or occur unilaterally. The duration of movement is typically between 1.5 and 2.5 seconds and varies in intensity from slight extension of the great toe to a triple flexion response. Patients frequently demonstrate a movement periodicity of between 20 and 40 seconds, although wide ranges of frequencies and muscle involvement have been reported. Movements are most pronounced in stage I and stage II of sleep, where they are often accompanied by K-complexes, and by increases in pulse and blood pressure (12).
This autonomic spike during the leg movements is increasingly scrutinized as it is postulated to account for the correlation between PLMS/RLS and risk of cardiovascular disease seen in some (13–16) but not all studies (17,18). Dopaminergics used to treat PLMS also blunt the autonomic response, as well as the actual movement (19). In contrast, K-complexes and arousals that usually precede the PLMS may persist even if PLMS are reduced (20). PLMS intensity and frequency lessen as sleep deepens. They may persist during REM sleep, but both their amplitude and frequency are significantly reduced. PLMS may result in arousals but are not generally associated with insomnia or excessive daytime sleepiness (21). Whether or not to treat isolated PLMS is debated.
RLS IN CHILDREN
RLS in children can be difficult to diagnose (22). Although some children report classic RLS symptoms that meet inclusion criteria, other complain of “growing pains,” (23,24) and some may present with an attention-deficit/hyperactivity disorder (ADHD) phenotype. Kotagal et al. reported that children with RLS have lower-than-expected serum ferritin levels and in most cases appear to inherit the disorder from their mother (25). Diagnostic criteria for RLS in children is less well validated but emphasizes supportive criteria such as a family history of RLS, sleep disturbances, the presence of PLMS, and typical descriptors used by children (26).
The exact relationship between RLS and ADHD is provocative but poorly understood. Children diagnosed with ADHD often have PLMS (27–30) and meet criteria for RLS (27). Children with ADHD have a higher prevalence of a parent with RLS (31). Children who meet diagnostic criteria for RLS also have higher rates of ADHD (32). Importantly, treatment of RLS/PLMS in children with ADHD with iron seems to help the ADHD (33,34), although treatment with levodopa (L-dopa) only inconsistently affects ADHD (35,36). Therefore, there is clearly some association between RLS and ADHD.
The treatment of pediatric RLS is much less studied. In open-label reports, anecdotally both oral and intravenous iron often improves symptoms, much more consistently than in adult RLS (33,34,37,38). A single controlled trial supports the use of L-dopa (36). Anecdotal experience also supports the use of gabapentin.
DIAGNOSTIC EVALUATION OF RLS
In most cases, only a simple evaluation is justified for clinically typical RLS. Serum ferritin and iron-binding saturation, for serum iron deficiency, and electrolytes for renal failure should be obtained. Nerve conduction velocities (NCV) and electromyogram (EMG) may be performed in cases without a family history of RLS, atypical presentations (i.e., sensations beginning in the feet or superficial pain), in cases that have a predisposition for neuropathy (i.e., diabetes), or when physical symptoms and signs are consistent with a peripheral neuropathy. If EMG/NCV abnormalities are found, they should be further evaluated. PSG evaluation is usually reserved for patients in whom the diagnosis is in doubt, in cases where PLMS are suspected to be severe and result in arousals, or if other sleep disorders are suspected.
There are several potential diagnostic dilemmas. Akathisia (discussed later) represents an inner sense of restlessness accompanied by an intense desire to move. These subjects do not typically complain of limb paresthesia. The restlessness is usually generalized, but may be most prominent in the legs. The condition is usually associated with the use of dopamine antagonist drugs. Painful legs and moving toes present with neuropathic leg pain associated with persistent, semirhythmic toe movements which cannot easily be reproduced volitionally, and may be only partially suppressed (39). Nocturnal leg cramps are a common, multifactorial disorder manifested by paroxysmal, disorganized spasms that usually involve the feet or calf muscles. The presentation is quite different from RLS, but patients may initially describe their RLS symptoms simply as “night cramps”, which can lead to misdiagnosis if a more extensive history is not taken. Body positional discomfort syndrome, where patients simply cannot find a comfortable position in which to lay; neuropathic or radicular leg pain, and PLMS are other conditions confused with RLS.
EPIDEMIOLOGY
Historically, epidemiologic studies of RLS were limited by the subjective nature of the disease, the lack of standardized diagnostic criteria, and the indolent onset of the condition. Since adaptation of standardized criteria, many large well-designed epidemiologic studies attest to the high prevalence of RLS. Studies in predominantly Caucasian populations consistently show that between 5% and 15% of people have RLS, which is clinically significant in 2% to 3% (40). In general, northern European countries demonstrated the highest prevalence, followed by Germanic/Anglo-Saxon, then Mediterranean countries. The prevalence tends to decline the farther east one progresses. Studies in Turkey and India show 2% to 3% prevalence, whereas this drops below 1% in Singapore. People from Africa have never been specifically studied but anecdotally African Americans only rarely present with RLS. It is unclear whether this represents a true lower prevalence, or differences in medical sophistication and referral patterns. Women usually have higher RLS rates. However, this female predisposition is lost in nulliparous women (41,42).
GENETICS OF RLS
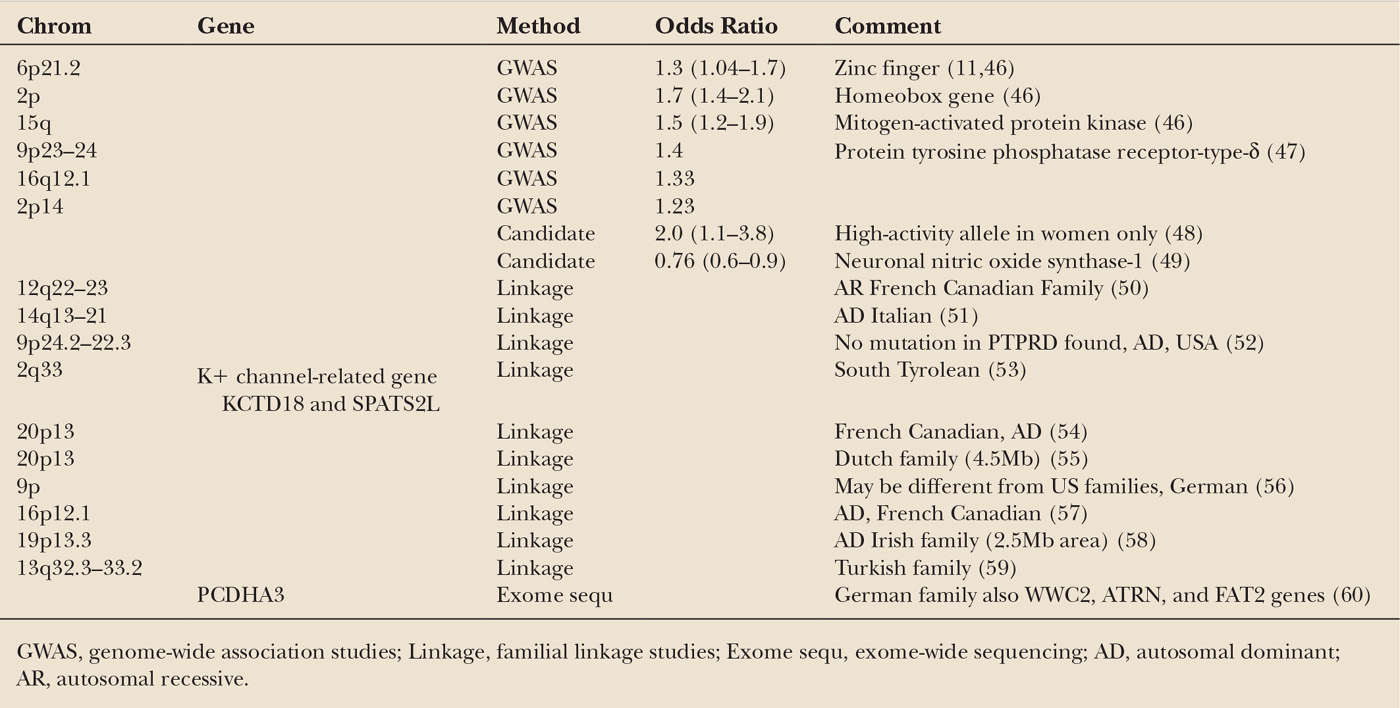
In 40% to 60% of cases, a family history of RLS can be found, although this is often not initially reported by the patient. Five Twin studies also show a very high concordance rate (43,44). Most pedigrees suggest an autosomal dominant (AD) pattern, (45) although an autosomal recessive (AR) pattern with a very high carrier rate is possible. Many linkages have been identified in traditional familial studies, but in no case has a specific gene mutation been identified (Table 34.1). A number of candidate genes, including those involved with dopamine metabolism, Parkinson’s disease (PD), neurodegeneration, and systemic iron regulation, have generally been unrevealing (61). To date, six risk-factor genes identified through genome-wide association studies have been published. Identification of the function and physiology of these genes is ongoing. Several of these are thought to be developmental genes and at least one is now associated with iron regulation. These appear to be most prevalent in Scandinavian populations, which have the highest prevalence of RLS. One recent study did not show that these genes were associated with Asian Uremic RLS patients (62). As with other common conditions, the genetics of RLS are complex and probably involve the presence of multiple at-risk genetic alleles.
PATHOPHYSIOLOGY OF RLS
Pathophysiologic studies show a number of abnormalities in patients with RLS; however, a complete understanding is far from complete. The most robust and consistent observation is reduced CNS iron stores, even in the setting of normal systemic iron studies. CSF ferritin is lower in RLS cases, (63) and specially sequenced MRI imaging studies show reduced iron stores in the striatum and red nucleus (64,65). CNS ultrasonography is also able to identify RLS based on reduced iron echogenicity in the substantia nigra (66,67). Most importantly, pathologic data in RLS autopsied brains show reduced H-ferritin staining, iron staining, and increased transferrin stains, but also reduced transferrin receptors (68). Researches also demonstrate reduced Thy1 expression, which is regulated by iron levels (69). Substantia nigra dopaminergic cells are not reduced in number, nor are there markers associated with neurodegenerative diseases, such as tau or α-synuclein abnormalities (70,71).
The reduced transferrin-receptor finding is especially important because globally reduced iron stores would normally upregulate transferrin receptors. Therefore, it appears that primary RLS has reduced intracellular iron indices associated with a perturbation of homeostatic mechanisms that regulate iron influx and/or efflux from the cell. Intracellular iron regulation is very complex and not entirely understood. Staining of RLS brains has shown reduced levels of iron regulatory protein type 1 (IRB-1) (68). This protein potentiates or inhibits (depending on feedback mechanisms involving iron atoms themselves) the production of ferritin molecules, which are the main iron storage proteins in the CNS as well as the periphery, and transferrin receptors, which facilitate intracellular iron transport. If and how reduced iron manifests the symptoms of RLS, or is a robust epiphenomenon, is not known.
CNS dopaminergic systems are also implicated in RLS. Dopamine agonists (DAs) acutely and robustly treat RLS. The normal circadian dopaminergic variation is also augmented in patients with RLS (72). However, dopaminergic brain imaging studies are inconsistent and show modest or no abnormalities (73–77). In summary, dopamine precursor studies show normal or reduced levels, dopamine transporter (DAT) studies have been normal or reduced, and dopamine receptor studies show reduced, normal, or increased activity. It should be noted that these mostly reflect activity in the striatum. There is no pathologic evidence of reduced dopamine cells or dopamine itself. Two studies using different ligands from the same group are interpreted as showing increased dopamine turnover (77). If fact, other indirect evidence also suggests increased dopamine turnover in RLS, to the point that some speculate RLS is a disease of hyperdopaminergic function (78). However, explanations for the dramatic and immediate effect of dopaminergics are not satisfactorily explained by this hypothesis.
There are several potential interactions between iron and dopamine systems. First, iron is a cofactor for tyrosine hydroxylase, which is the rate-limiting step in the production of dopamine. Iron chelation reduces DAT protein expression and activity in mice (79). Human CSF studies, however, have failed to demonstrate reduced dopaminergic metabolites (80,81). Second, iron is a component of the dopamine type-2 (D2) receptor. Iron deprivation in rats results in a 40% to 60% reduction of D2 postsynaptic striatal but not spinal cord receptors (82–84). The effect in the striatum is quite specific, as other neurotransmitter systems, including D1 receptors, are not affected. Third, iron is necessary for Thy1 protein regulation. This cell adhesion molecule, which is robustly expressed on dopaminergic neurons, is reduced in brain homogenates in iron-deprived mice (85) and in brains of patients with RLS (69). Thy1 regulates vesicular release of monoamines, including dopamine (86). It also stabilizes synapses and suppresses dendritic growth (87).
Another puzzle remains regarding identification of a specific anatomy culpable for RLS. The subcortex is implicated in studies of RLS onset after stroke, and (88) functional involvement of the medial thalamus, cerebellum, and brain stem have also been implicated using functional MRI techniques (89,90).
Spinal cord lesions often result in RLS and PLMS. Specifically, involvement of the little-studied diencephalospinal dopaminergic tract, originating from the A11–A14 nuclei, might explain some RLS features. It is involved in antinociception, is near circadian control centers, and would explain why legs are involved more than arms. A preliminary animal model with A11 lesions demonstrated increased standing episodes, which improved after the administration of ropinirole, a DA (91). Subsequent studies of this model in mice, with and without dietary iron deprivation, also demonstrate increased movement, as measured in laser-marked cages, in the lesioned animals (92). This hyperkinesis is normalized by D2 agonists such as ropinirole and pramipexole, but worsened by the D1 agonist SKF.
Opioid pathways are implicated by clinical improvement seen with narcotics and pathologic data that show reduced number of β-endorphin positive cells (37.5%, p = .006, effect size 2.16) and Met-enkephalin positive cells (26.4%, p = .028, effect size 1.58) in six RLS patients compared to six controls (93). Dopamine activity was normal in this study.
Afferent systems are also implicated (94,95). Stiasny-Kolster reported that pin-prick pain ratings (static hyperalgesia) in RLS patients were significantly elevated in the lower limb, whereas pain to light touch (allodynia = dynamic mechanical hyperalgesia) was normal. They felt this type of hyperalgesia was probably mediated by central sensitization to A-δ fiber high-threshold mechanoreceptor input, a hallmark sign of the hyperalgesia type of neuropathic pain.
Recently, (1)H MRS showed increased glutaminergic activity in the thalamus of RLS patients (96). This correlated with increased arousal but not PLMS.
“SECONDARY RLS”
Despite the appropriate attention given to RLS genetics, many suffer from RLS without any identifiable highly penetrant genetic pattern. Patients without a positive family history are classified as either primary RLS, if no other explanation is found, or secondary RLS, if they concurrently have a condition known to be associated with RLS.
The most common diseases associated with RLS include renal failure, iron deficiency, neuropathy, myelinopathy, pregnancy, multiple sclerosis (MS) (97), and possibly PD and essential tremor (ET). There are many reports; some evidence to support an association of RLS with some genetic ataxias (98,99), fibromyalgia (100), and rheumatologic diseases (101–103). Many of additional reported associations are not replicated or tenuous.
Numerous forms of neuropathy, including diabetic, alcoholic, amyloid, motor neuron disease, poliomyelitis, and radiculopathy, have been seen at higher-than-expected frequency in patients presenting with RLS (5,104–109). In contrast, series evaluating RLS in populations presenting with neuropathy have not shown a particularly high prevalence of RLS, usually ranging from 5% to 10%, similar to the general population (108,109). In one of the best controlled studies, Hatten et al. found that a larger percentage of neuropathy patients endorsed RLS on screen questionnaires (18.4% versus 6.1%), but when interviewed, this narrowed to 12% versus 8% of controls. In general, axonal neuropathies are more associated than demyelinating types. It is likely and intuitive that deafferentation increases risk for RLS, but this relationship remains unclear.
The spinal cord is implicated in the pathogenesis of RLS (110), and cases of RLS and PLMS are seen after transient or permanent spinal cord lesions. Traumatic spinal cord lesions (111,112), neoplastic spinal lesions (113), demyelinating or postinfectious lesions (114–116), and syringomyelia (117) all can precipitate RLS and PLMS. Spinal cord blocks used for anesthesia also may cause or exacerbate RLS (118,119). In one study of 161 subjects without any history of RLS, 8.7% developed RLS immediately after spinal anesthesia (118).
Uremia secondary to renal failure is strongly associated with RLS symptoms. Many series report a 20% to 57% prevalence of RLS in renal dialysis patients; however, only a minority of uremic patients volunteer RLS symptoms unless specifically queried. Numerous risk factors have been identified in individual studies but none consistently. The RLS seen in dialysis patients is often severe and associated with high PLMS indexes. Both RLS and PLMS have also been associated with increased mortality in the dialysis population (120–122). Overall, dialysis does not improve RLS. In fact, one study suggested that RLS correlated with greater dialysis frequency (123). However, patients who receive a successful, but not unsuccessful, kidney transplant usually experience dramatic improvement in RLS within days to weeks (124–126). The degree of symptom alleviation appears to correlate with improved kidney function.
Reduced CNS iron is possibly implicated in all cases of RLS. It is intuitive to suggest that reduced body stores of iron could also result in low CNS intracellular iron and also cause RLS symptoms. A series of reports have associated low serum ferritin levels with RLS (63,64,127–131). Anemia has not been independently associated with idiopathic RLS; however, blood donors do frequently develop RLS symptoms (129,132). Serum ferritin is the best indicator of low iron stores and the only serum measure to consistently correlate with RLS. It should be noted that ferritin is an acute phase reactant and that “normal” levels increase with age. Therefore, a “normal”-range ferritin does not exclude actual iron deficiency.
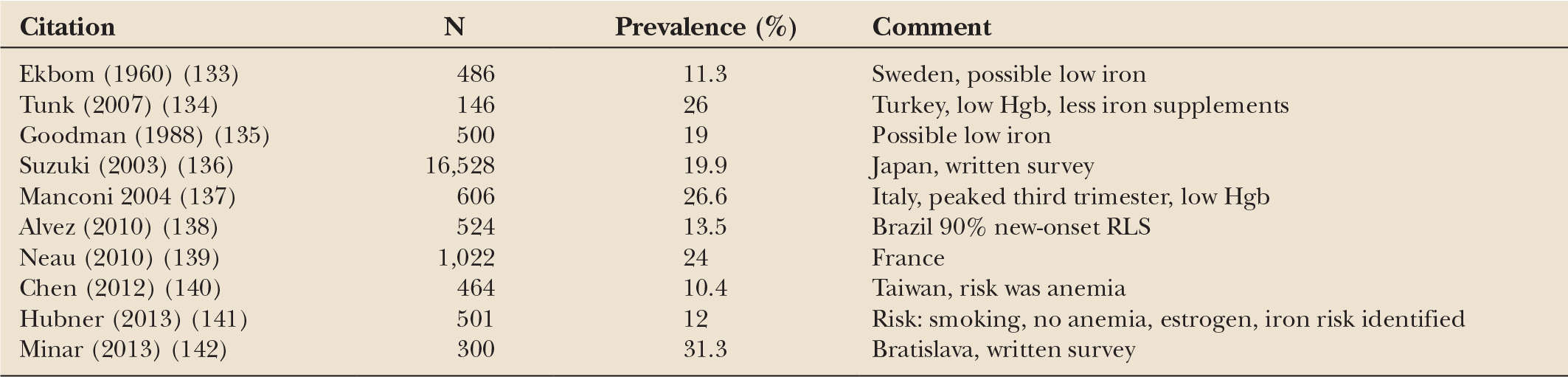
The development of RLS during pregnancy has long been recognized (Table 34.2) (133,135,143). Manconi et al. (137) carefully evaluated risk factors for RLS in 606 pregnancies. They reported that 26% of these women suffered from RLS, usually in the last trimester. The authors could find no significant differences in age, pregnancy duration, mode of delivery, tobacco use, the woman’s body mass index, baby weight, or iron/folate supplementation in those with RLS. Hemoglobin, however, was significantly lower in the RLS group, and plasmatic iron tended to be lower, compared to those without RLS. Several other studies have also correlated RLS with anemia before (144) or during pregnancy (134). Increased estradiol, seen mostly in the last trimester, has also been postulated to cause RLS (134,145). Transient RLS during pregnancy does appear to increase the risk of RLS later in life (146).
Series of patients with MS usually show high rates of RLS. One meta-analysis of 24 studies reported a fourfold increase compared to controls (147). RLS has been associated with primary progressive MS, spinal cord lesions, longer duration of MS, and worse disability (97).
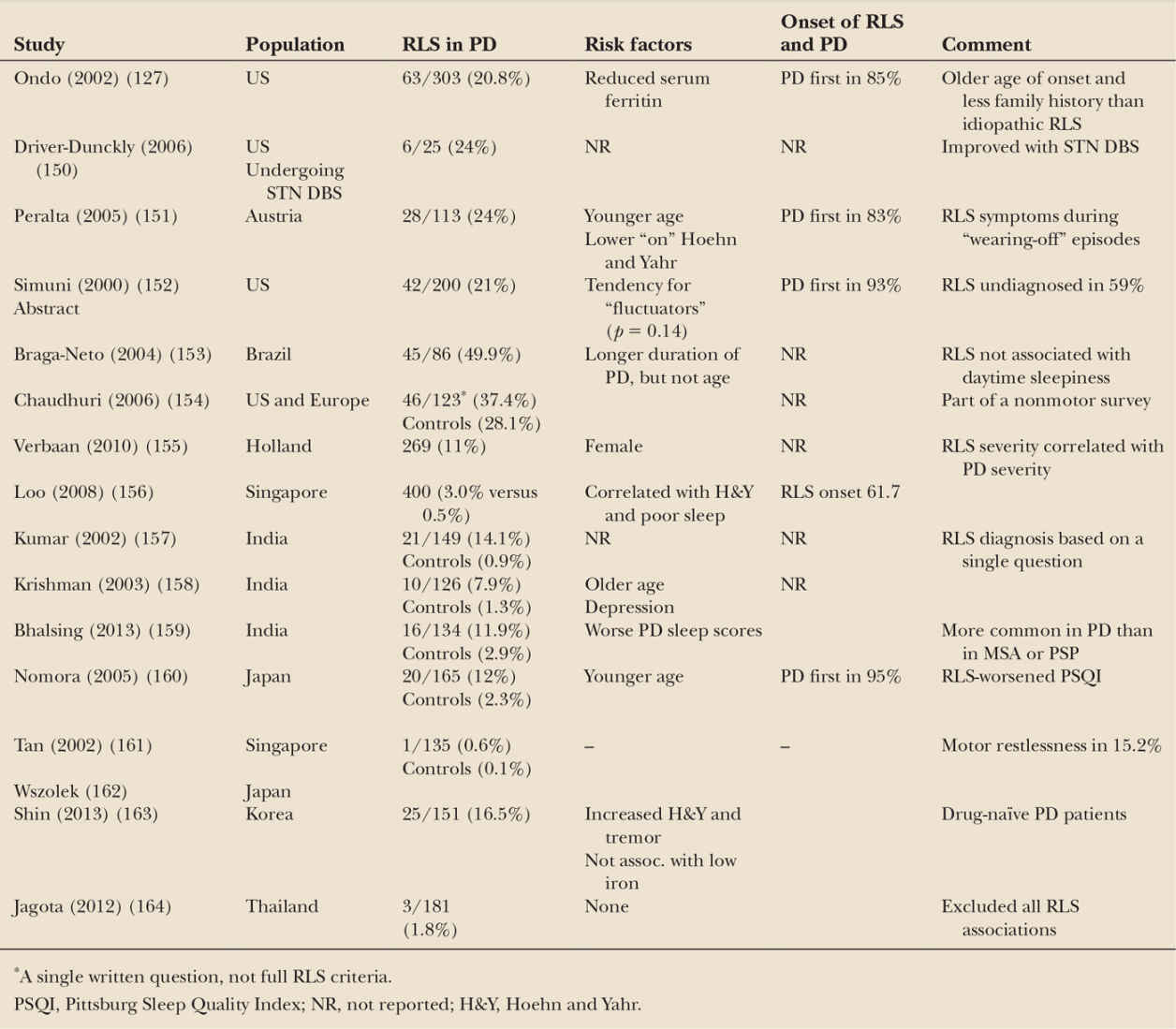
PD and RLS both respond to dopaminergic treatments, both show dopaminergic abnormalities on functional imaging (75,148), and both are associated with PLMS (149). However, we now know that the pathology of the two dopaminergically treated diseases are very different, and in regard to iron accumulation, actually quite opposite (71). Several surveys, especially in Caucasians, report a greater-than-20% prevalence of RLS in PD populations (Table 34.3). In a survey of 303 consecutive PD patients, we found that 20.8% of all patients with PD met the diagnostic criteria for RLS. Only lower serum ferritin was associated with RLS (127). Asian populations, which have less RLS in general, also have lower rates of RLS in their PD populations, but these are still consistent higher than their control populations.
Despite this high number of cases, there are several caveats that tend to lessen its clinical significance. The RLS symptoms in PD patients are often ephemeral, usually not severe, and can be confused with other PD symptoms such as wearing-off dystonia, akathisia, or internal tremor. They do not correlate with daytime sleepiness, and PD precedes the RLS in most cases. There is no compelling evidence that RLS becomes PD at a later date. If fact, we found that patients with long-standing RLS or with a family history or RLS, who did go on to develop PD, did so at a later age than a control group of PD patients without prior RLS. In contrast, using a large medical record survey in Japan, Wong et al. (165) found that symptoms of severe RLS were associated with the development of PD within 4 years, but not later, suggesting that RLS may be an early premotor feature of PD. The pathophysiology of RLS symptoms in PD is not known and may or may be different from idiopathic RLS.
ET is associated with RLS in one study (166). Patients in the ET/RLS group almost always present with ET, often severe. Interestingly, they have family histories of both suggesting a common genetic origin.
Several medications are known to exacerbate existing RLS or possibly precipitate RLS themselves. The most notable of these include antihistamines, dopamine antagonist, including many antinausea medications, mirtazapine, and possibly tricyclic antidepressants and serotonergic reuptake inhibitors (SSRIs). Sedating antihistamines, which cross the blood–brain barrier, most robustly exacerbate RLS. Most RLS patients have experienced this, especially since many are marketed as sleep aids. SSRIs are more associated with PLMS.
TREATMENT OF RLS
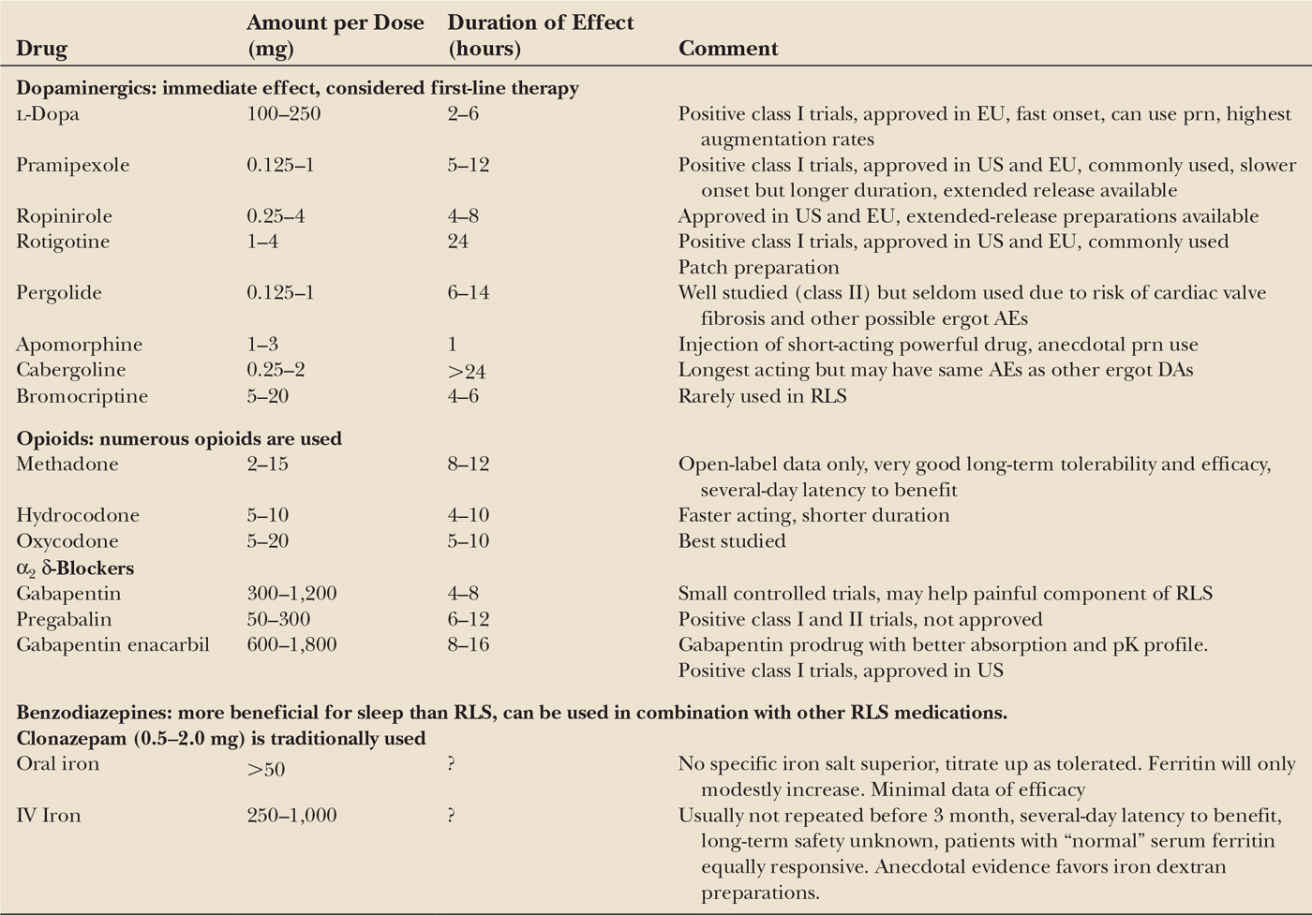
The development of validated rating scales and standardized diagnostic criteria have vastly improved the quality of RLS treatment trials. Multiple medications have demonstrated efficacy in well-designed trials, especially dopaminergics, α2 δ-ligands, opioids, and iron (Table 34.4) (167,168). With the possible exception of iron, all are felt to provide only symptomatic relief, rather than any “curative” effect. Therefore, treatment should only be initiated when the benefits are felt to justify any potential side effects and costs. Treatment decisions also need to consider the chronicity and general progressive course of RLS. Over time, both dosing and drug changes may be required to maximize benefit and minimize the risk of side effects.
DAs are the best investigated consistently effective treatments for RLS. The improvement is immediate and often very dramatic. No direct evidence favors any particular DA. Ropinirole (169–171), pramipexole (172–174), and rotigotine patches (175,176) are the best studied. Pergolide (169,177,178), bromocriptine (179), apomorphine (180), cabergoline (181), and lisuride (182) are also effective. The dopamine precursor L-dopa also effectively treats RLS; however, several comparative studies have favored DA over L-dopa (183–185).
PSG studies of DA consistently demonstrate dramatic improvement in PLMS, but modest or no improvement in other sleep parameters. Acute adverse events in RLS DA studies are generally milder than in PD studies, perhaps owing to the lower dose or differences in the disease state. Hallucinations and hypotension rarely occur in RLS, and daytime sedation may lessen rather than increase. Nausea remains the most common adverse event.
Immediate-release oral DA works best if administered at least 90 minutes before the onset of symptoms. The effect is immediate, so titration to the smallest effective dose can be fairly rapid. Based on pharmacokinetics, many people may benefit from more than one dose, despite the formal indications, which recommend dosing 1 to 3 hours before bed. Extended-release preparations of pramipexole and ropinirole are also very effective but have not been formally studied.
The long-term use of DA for RLS is more problematic, as some subjects develop tolerance and others can develop very problematic augmentation (186). Augmentation is defined by an earlier phase shift of symptom onset, an increased intensity of symptoms, increased anatomic involvement, or less relief with movement (187). L-Dopa has the worst augmentation, often within 6 months, and is much less used because of this problem (188). Augmentation with DAs is modest at 1 year (2%–9%) but seems to increase linearly over time (189–192). Risk factors for augmentation have been inconsistent but include lower serum ferritin, higher dose of a dopaminergic, worse RLS, a family history of RLS, and absence of neuropathy (188,193,194).
< div class='tao-gold-member'>