Fig. 20.1
Preoperative thin-slice temporal bone CT. (a) Note the close relationship of the cochlea (black arrow), vestibule (white arrowhead), and internal auditory canal (asterisk). The emissary vein (black arrowhead) is seen in the way to the sigmoid sinus. (b) The relationship of the endolymphatic duct (white arrow) to the labyrinth structures (white arrowhead) and internal auditory canal (asterisk). (c) Normal position of the jugular bulb (black arrow) 4 mm below the internal auditory canal (asterisk)
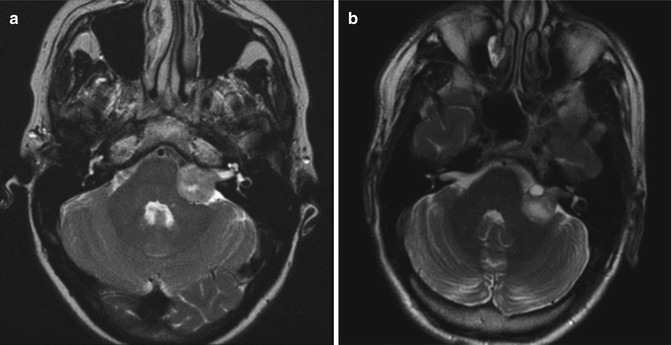
Fig. 20.2
Preoperative T2-weighted MRI showing vestibular schwannomas of similar sizes, however with different intrameatal extensions. (a) The tumor does not reach the fundus of the internal auditory canal. Note the CSF collection between the tumor and the fundus. (b) The tumor reaches the fundus filling completely the internal auditory canal
Cerebral angiography is required particularly in meningiomas. Eventually, embolization is needed to diminish intraoperative bleeding and reduce operative morbidity.
20.3 Retrosigmoid Approach to the Posterior Fossa
20.3.1 Surgical Technique
The operation is carried out with general anesthesia in the supine, park-bench, or semisitting position [14]. The semisitting position is the authors’ preference. However, the supine position is preferred in cases of microvascular decompression or patent foramen ovale. At surgery, bilateral SEP and electrophysiological monitoring of the ipsilateral VII and VIII cranial nerves are performed for upper CPA lesions. For large tumors and lower CPA lesions, the electrophysiological records of the caudal cranial nerves are also monitored.
The head is fixed with the Mayfield frame in the form that the single pin is placed on the side of the lesion around the linea temporalis and anterior to the external auditory canal. The paired pins are applied also around the linea temporalis on the contralateral side. The patient is then placed in a semisitting position with the head hyperextended, turned 30° toward the affected side, and flexed. The legs are raised to or above the level of the heart and the knees are slightly flexed (Fig. 20.3). Thus, the venous pressure could be raised and, therefore, the risk of air embolus is reduced. Bilateral SEPs are made continuously during positioning. In the case of changes in SEP’s latency or amplitude, the surgeon should be warned in order to correct the patient’s position. Precordial Doppler device is attached to recognize air embolus. Recently, air embolus has been monitored by the help of transesophageal echocardiography.
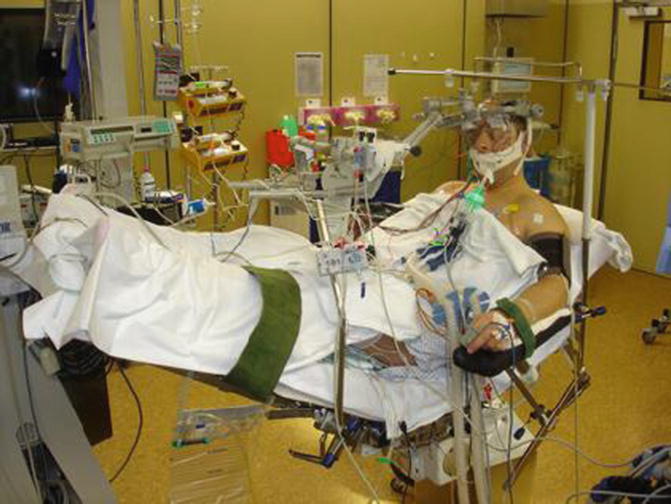

Fig. 20.3
View of the Mayfield fixation and surgical position. The patient is in the semisitting position with the legs at the level of the heart
The hair is shaved to ease identification of the surface anatomy. A slightly curved skin incision is drawn with a skin marker extending superiorly 2 cm behind the pinna, passing through the asterion and terminating 1–2 cm medial to the mastoid tip (Fig. 20.4). The inferior border of the skin incision depends on the need of caudal extension. No local anesthetic is used. The skin flap is elevated with the periosteum and held with a skin retractor. The neck muscles are divided in line with the skin incision and retracted with a self-retaining Leyla retractor.
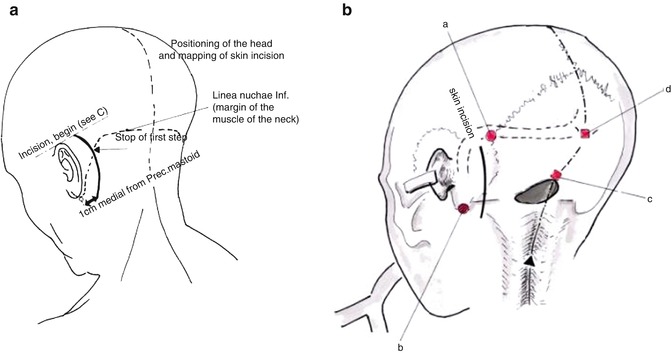

Fig. 20.4
The surface landmarks are examined. (a) The skin incision extends from 2 cm behind the pinna to 1–2 cm medial to the mastoid tip. (b) The course of the transverse and sigmoid sinus, as well as the transverse-sigmoid sinuses’ transition, is drawn (black dotted line) (a asterion, b mastoid tip, c opisthion, d inion) (Courtesy of Prof. Wolfgang Seeger)
The burr hole is performed below the asterion and then a suboccipital craniectomy is carried out with rongeurs toward the transverse and sigmoid sinus and the squamous part of the occipital bone. Craniotomy is also an option, especially for young patients. The asterion and the posterior part of the parietomastoid suture allow a good estimate of the extracranial projection of the transverse-sigmoid sinuses’ transition [22] and, therefore, constitute the superolateral limit of the craniectomy [13, 22] (Fig. 20.5). Virtual reality in image guidance may also be used to recognize in real time the position of the dural sinuses and their relationship to bone landmarks, thereby facilitating surgical planning [23]. Usually, a suboccipital craniectomy of about 3–4 cm in diameter is enough to approach the CPA.
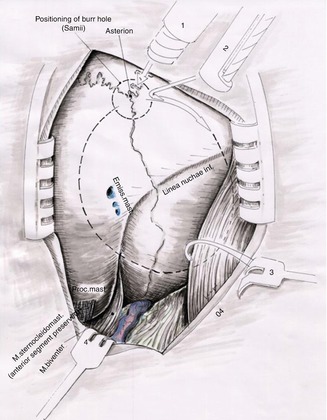

Fig. 20.5
Note the placement of the burr hole nearby the left asterion (1). The black dotted line corresponds approximately to the area of suboccipital craniectomy (2) craniotome, (3 and 4) skin retractors (Courtesy of Prof. Wolfgang Seeger)
High-speed diamond drill is used to expose the borders of the transverse sinus, the transverse-sigmoid sinuses’ transition, and the sigmoid sinus (Fig. 20.6). Continuous irrigation provides cooling during drilling. Bone wax is used to pack the mastoid air cells and the emissary vein. Care should be taken with the emissary vein when operating on semisitting position in the way that its laceration may precipitate air embolus. When a large emissary vein is detected by preoperative temporal bone thin-slice CT, we prefer to skeletonize the vein with a fine diamond burr and coagulate it under direct view. Smaller craniectomies are accomplished if microvascular decompression is planned, directed to the upper or lower CPA [12, 15, 22, 24].
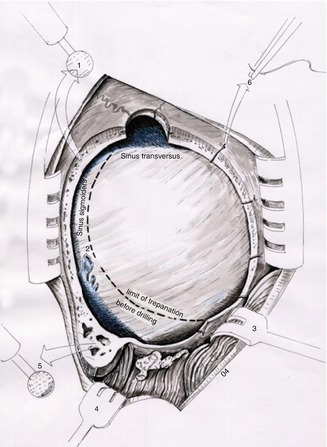

Fig. 20.6
Schematic view of the suboccipital craniectomy before and after drilling. The black dotted line indicates the limit of bone resection performed with rongeurs. High-speed diamond drill (1 and 5) is used to enlarge the craniectomy bringing into view the transverse-sigmoid sinuses’ transition, as well as the transverse and sigmoid sinuses, (2) emissary vein, (3 and 4) skin retractors, (6) dural sutures (Courtesy of Prof. Wolfgang Seeger)
Supporting arm rests are attached to the operating table providing more comfort and accuracy for the surgeon. The dura is opened in a C-shaped medially based fashion along the transverse and sigmoid sinus [1, 12] under the magnification of the operating microscope. The opening is performed some millimeters away from the sinuses to allow adequate closure [1, 12]. Auxiliary oblique incisions at the angles of the primary incision increase the surgical view [12], but it is not routinely necessary. Three or four tack-up sutures are placed for further enhancement of the surgical view, thereby reducing the need for cerebellar retraction (Fig. 20.7). Solely upper or lower incisions are performed according to the underlying pathology encountered in the upper or lower CPA, respectively [12].
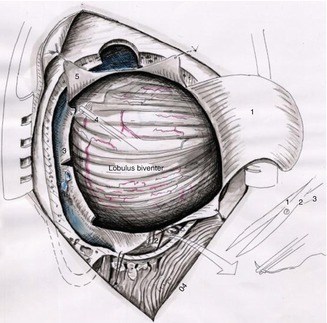

Fig. 20.7
Schematic view of retrosigmoid approach showing the C-shaped dural flap (1) and the tack-up sutures (5). Eventually, small oblique incisions are accomplished to increase surgical exposure (3), (2 and 4) tack-up dural sutures for enhancement of the surgical view (Courtesy of Prof. Wolfgang Seeger)
With a narrow brain retractor and a fine bayonet forceps, the surgeon opens the CSF cisterns. We use cotton strips to protect the cerebellum against lacerations. Thus, cerebrospinal fluid (CSF) is withdrawn then relaxing the cerebellum (Fig. 20.8). Further brain relaxation is achieved with mannitol administered intravenously at the time of skin incision. Thereafter, the brain retractors have a more protective effect without significant compression on the cerebellum. Oblique and smooth movements of the brain spatula on the cerebellum are preferred than longitudinal stretching through the facial-vestibulocochlear nerve bundle in order to avoid damage to the vestibulocochlear nerve [21, 25–27]. Besides, for vestibular schwannoma and upper CPA tumors, the smooth movements of the brain spatula are also required to prevent the occurrence of trigeminocardiac reflex [28–30]. The foramen of Luschka and the lateral recess of the fourth ventricle are reached with a slight elevation of the cerebellar flocculus. The next steps are determined for the underlying condition and are described below.
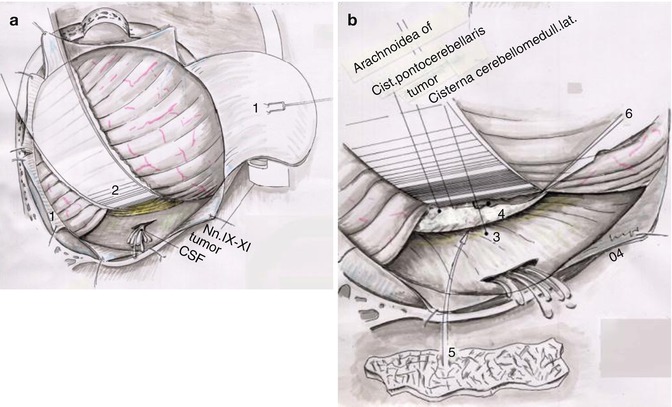

Fig. 20.8
Schematic view representing the opening of the lateral cerebellomedullary cistern. (a) By elevating the cerebellum using a narrow brain spatula, the surgeon opens the CSF cistern with a fine bayonet forceps. In (b), the view is amplified demonstrating one of the most important steps of the surgical approach (1) retracted dura, (2) brain retractor, (3) lateral cerebellomedullary cistern, (4) cerebellopontine angle cistern, (5) after opening of CSF cisterns, a small cotonoid is placed over caudal cranial nerves, (6) surgical blade (Courtesy of Prof. Wolfgang Seeger)
One advantage of the semisitting position is spontaneous drainage of CSF and blood that allied to suction irrigators with saline solution which provides a clean surgical field, thereby reducing the time of dissection. Recently, we have found such “gravitational” advantage not so essential for small intracanalicular vestibular schwannomas. These patients are now operated on the supine position at our department. At the end of the procedure, jugular vein compression is done by the anesthesiologist to search for possible venous bleeding, especially in the semisitting position. Hemostasis is achieved with utmost care.
The dural flaps are gathered with non-resorbable single sutures and then a continuous suture closes the dural opening in a watertight fashion. Dural patch is seldom needed due to the C-shaped opening. Cranioplasty is performed with methyl methacrylate. The mastoid cells are covered with muscle and fibrin glue. The neck muscles are sutured back, respecting the anatomical layers. No drains are used. A compressive bandage is used after the skin closure.
20.3.2 Clinical Applications
20.3.2.1 Microvascular Decompression
After the standard craniectomy and dural opening, the superolateral surface of the cerebellum is inspected for small bridging veins. When encountered, these veins are coagulated and transected. The brain retractor is applied at the edge of the petrotentorial junction accompanying the superior petrosal sinus for trigeminal nerve decompression. Care should be taken with the petrosal vein. Although several authors advocate its ligature without additional morbidity [15, 24], we, on the other hand, spare the petrosal vein whenever possible due to complications that have been reported in previous communications [31, 32].
For facial nerve decompression, the caudal cranial nerves should be identified first, and then the dissection is taken upward toward the brainstem to avoid stretching of the facial-vestibulocochlear nerve bundle.
Anatomical [33] and clinical studies [34, 35] of endoscope-assisted microvascular decompression for trigeminal neuralgia and facial hemispasm have demonstrated feasibility of this technique in obtaining similar results to the standard microvascular decompression. We, however, do not use this method routinely for the management of such pathologies.
20.3.2.2 Vestibular Schwannoma
The CPA and the tumor are exposed after opening the lateral cerebellomedullary cistern with a fine bayonet forceps and the cerebellum slightly lifted with a narrow brain retractor attached to a self-retaining Leyla retractor (Fig. 20.9). We prefer initially to open the internal auditory canal (IAC) regardless of the tumor size due to elevation of intrameatal pressure [36, 37]. This impairs the blood supply of the cochlear nerve and labyrinth [36, 37]. Thus, the ischemic environment of the inner ear is further worsened by traction, tumor debulking, dissection, or the hypotension precipitated by the trigeminocardiac reflex [29], thereby decreasing the chances of hearing preservation. During the entire surgery and particularly tumor dissection, the mean arterial blood pressure is maintained around 90 mmHg to avoid the negative impact of hypotension on auditory function [29].
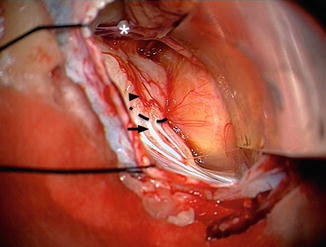

Fig. 20.9
Intraoperative view showing a large vestibular schwannoma (Type IVa) on the left side after cerebellar retraction with a narrow curved brain retractor. The petrosal vein is observed in the way to the superior petrosal sinus (asterisk). The Tübingen line is easily identified (black dotted line). The first step is to recognize several vertical dural foldings (black arrow). The transition zone to the smooth dural area at the suprameatal tubercle (black arrowhead) is the Tübingen line. It corresponds exactly to the inferior border of the internal auditory canal
A C-shaped dural opening of about 1 × 1 cm is performed based on the posterior lip of the IAC and directed superolaterally. In cases of large and giant vestibular schwannomas, the identification of the IAC is difficult or impossible since the posteriormost part of the tumor blocks the surgical view to the petrous part of the temporal bone [38]. With increasing experience, the first author (M.T.) has noted in 300 consecutive cases the relationship of a dural landmark in the petrous bone and the inferior border of the IAC. This dural landmark is called the Tübingen line. This is a slightly curvilinear line observed in the transition between the dural-folded area adjacent to the vestibular aqueduct and the smooth area, in which the dura is tightly adherent to the posterior surface of the petrous bone (Figs. 20.9 and 20.10). The Tübingen line is a very straightforward and reliable method for IAC identification. This was confirmed in anatomical specimens [38]. When the Tübingen line cannot be recognized, which occurs in <2 % of the cases, IAC identification is based on microsurgical knowledge, preoperative bone-windowed CT, and the surgeon’s experience [39]. Additionally, navigation-guided opening of the IAC was attempted to direct drilling due to the anatomical variability of the labyrinth structures [39].
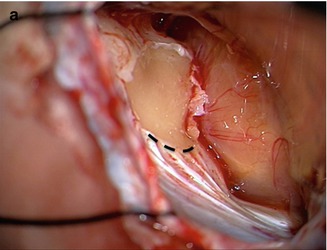
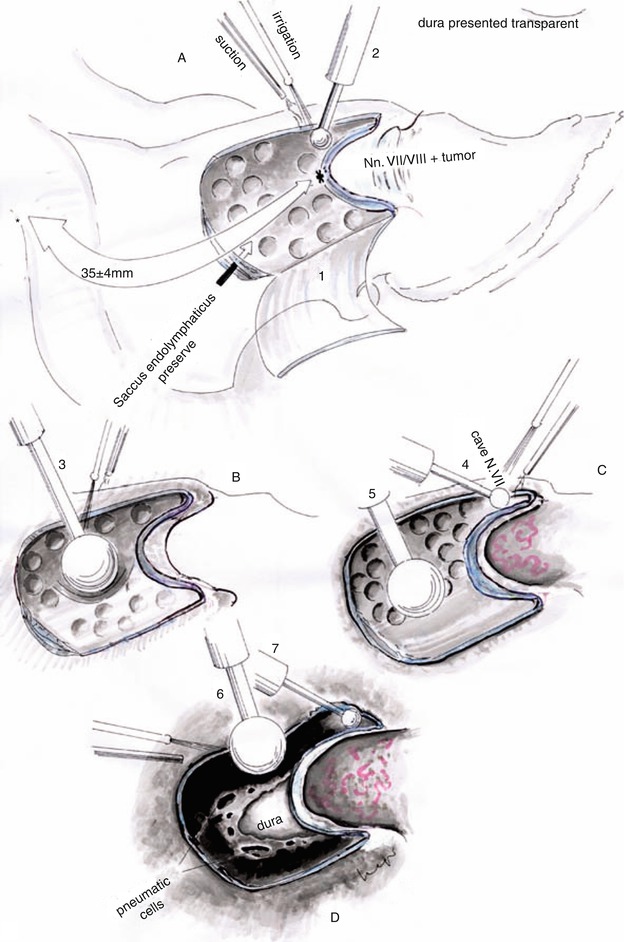


Fig. 20.10
(a, b) Intraoperative view of the dural incision directed superolaterally precluding endolymphatic duct injury. The Tübingen line is the inferior line of the dural incision (black dotted line). Schematic view of the IAC’s opening by using progressive smaller diamond burrs. The roof of the IAC is an important anatomical landmark guiding the bone removal (Courtesy of Prof. Wolfgang Seeger)
We begin with a large cutting burr that is progressively replaced for smaller diamond burrs as the IAC is approached (Fig. 20.10). Irrigation is used for cooling. The roof of the IAC is the best anatomical landmark for removal of the posterior wall [12]. The length of drilling is tailored by preoperative bone-windowed CT, but usually 8 mm is sufficient for adequate exposure of the intrameatal contents. Further drilling increases the risk of labyrinth damage.
The IAC dura is opened longitudinally (Fig. 20.11). Once the tumor is identified, piecemeal debulking is started with microscissors and tumor forceps. A short hook is used to create the arachnoid plane between the tumor and the nerves. Then, a long hook is introduced from medial to lateral to palpate the IAC fundus and to remove the tumor. Pulling the tumor medially is avoided due to stretch of the tiny cochlear fibers. En bloc removal is possible for small tumors. Usually, the facial nerve is displaced anterosuperiorly and the cochlear nerve anteroinferiorly. Frequently, bipolar stimulation is used to identify the facial nerve into the IAC (Fig. 20.12).
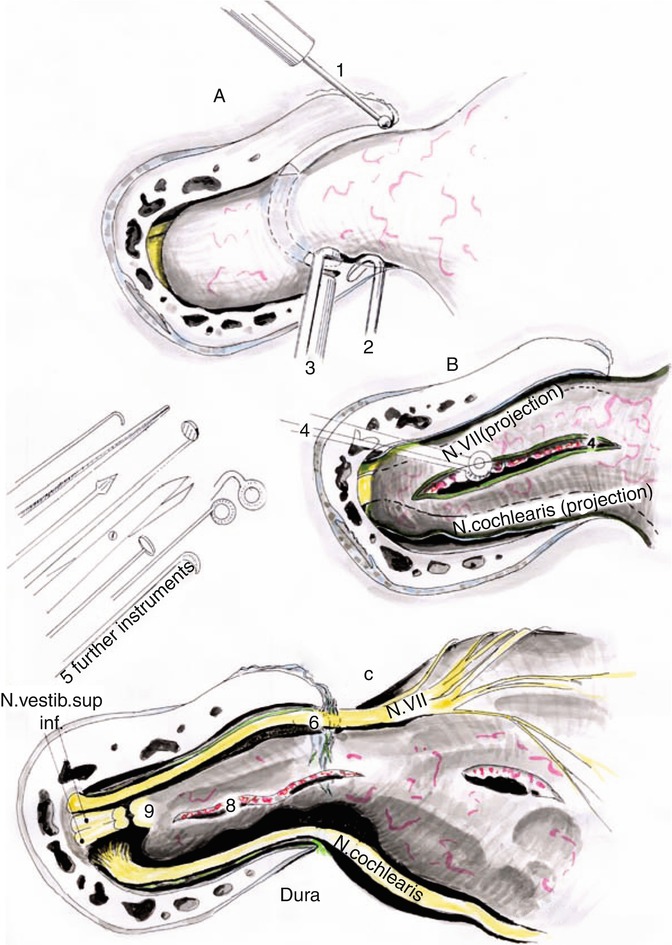
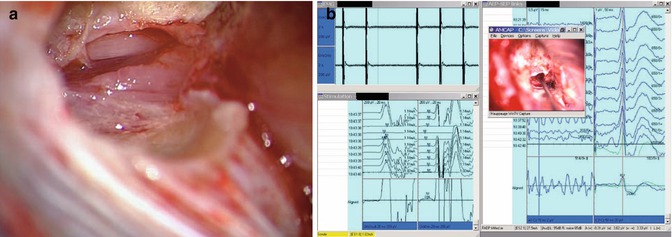

Fig. 20.11
(a) Following bone removal the tiny bones attached to the edges of the IAC are removed by using a small diamond burr (1) or fine hooks (2). (b) The dura is opened longitudinally and tumor debulking is initiated intrameatally (4). (c) Thereafter, the facial, cochlear, and vestibular nerves are exposed (c), (3) fine roungers, (5) microsurgical instruments, (6) facial nerve (N. VII), (8) intrameatal tumor, (9) transected vestibular nerves (Courtesy of Prof. Wolfgang Seeger)

Fig. 20.12
Intraoperative view following piecemeal tumor debulking. The facial nerve comes into view, which is usually displaced anterosuperiorly (left). Identification of the facial nerve within the IAC through bipolar stimulation is sometimes required (right)
Thereafter, dissection is directed to the CPA. The tumor capsule is opened and used to develop the arachnoid plane. The tumor is progressively debulked with microscissors, tumor forceps, and ultrasonic aspirator. When the capsule is relaxed, we use a tumor forceps to hold the tumor and a fine bayonet forceps to create the arachnoid plane (Fig. 20.13). Then, debulking and dissection are subsequently alternated in a stepwise manner. In tumors with too soft or too hard consistency, as well as in cystic vestibular schwannomas, the plane development may be difficult to achieve. The site of maximal adhesion, normally at the IAC, is prepared at the end. The branch of the vestibular nerve is transected and the tumor is removed without jeopardizing the facial and cochlear nerves. To assure the completeness of removal, an endoscope may be introduced to examine the IAC fundus and remove eventual tumor rests [40].
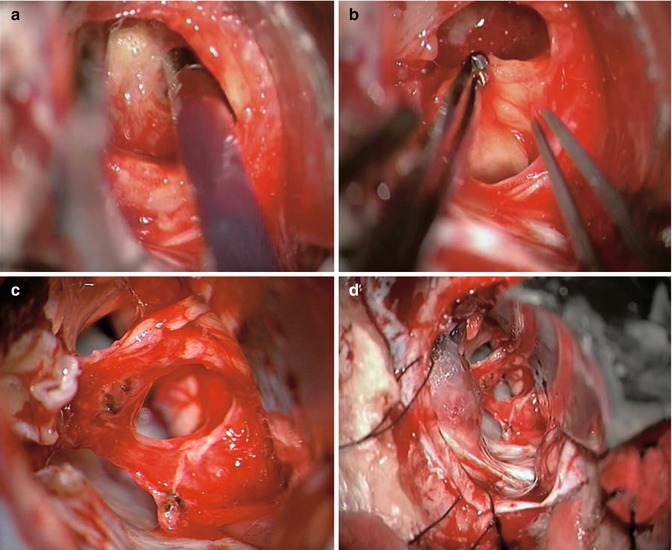

Fig. 20.13




Intraoperative view of a large vestibular schwannoma. The tumor is debulked with an ultrasonic aspirator (upper left). While the capsule is relaxed, the arachnoid plane is developed by using two forceps, one tumor forceps to hold the tumor and a fine bayonet forceps to create the plane. Note the caudal cranial nerves (upper right). Final aspect following complete tumor resection. The facial, superior vestibular, and cochlear nerves are anatomically preserved (lower left). After meticulous hemostasis, the IAC is sealed with a piece of muscle and fibrin glue (lower right)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








