Functional Anatomy of the Somatic Sensory Pathways
Sensory Changes & Their Significance
Distinction Between Organic & Psychogenic Sensory Disturbances
Idiopathic Inflammatory Neuropathies
Acute Idiopathic Polyneuropathy (Guillain-Barré Syndrome)
Chronic Inflammatory Demyelinating Polyneuropathy
Metabolic & Nutritional Neuropathies
Infective & Granulomatous Neuropathies
Neuropathies In Vasculitis & Collagen Vascular Disease
Systemic Necrotizing Vasculitis
Granulomatosis With Polyangiitis (Wegener Granulomatosis)
Scleroderma & Mixed Connective-Tissue Disease
Neoplastic & Paraproteinemic Neuropathies
Compression & Infiltration by Tumor
Drug-Induced & Toxic Neuropathies
Hereditary Motor & Sensory Neuropathies
Hereditary Sensory & Autonomic Neuropathies
Hereditary Neuropathy with Liability to Pressure Palsies
Upper Limb Entrapment Syndromes
Lower Limb Entrapment Syndromes
Lateral Femoral Cutaneous Neuropathy
Compressive & Traumatic Lesions
Subacute Combined Degeneration
APPROACH TO DIAGNOSIS
In order to interpret the history and clinical signs of patients with disorders of somatic sensation, the functional anatomy of the sensory components of the nervous system must be understood. As used here, somatic sensation refers to the sensations of touch or pressure, vibration, joint position, pain, and temperature, and to more complex functions that rely on these primary sensory modalities (eg, two-point discrimination, stereognosis, graphesthesia); it excludes special senses such as smell, vision, taste, and hearing.
FUNCTIONAL ANATOMY OF THE SOMATIC SENSORY PATHWAYS
The sensory pathway between peripheral tissues (eg, skin or joints) and the cerebral cortex involves three neurons and two central synapses (Figure 10-1).
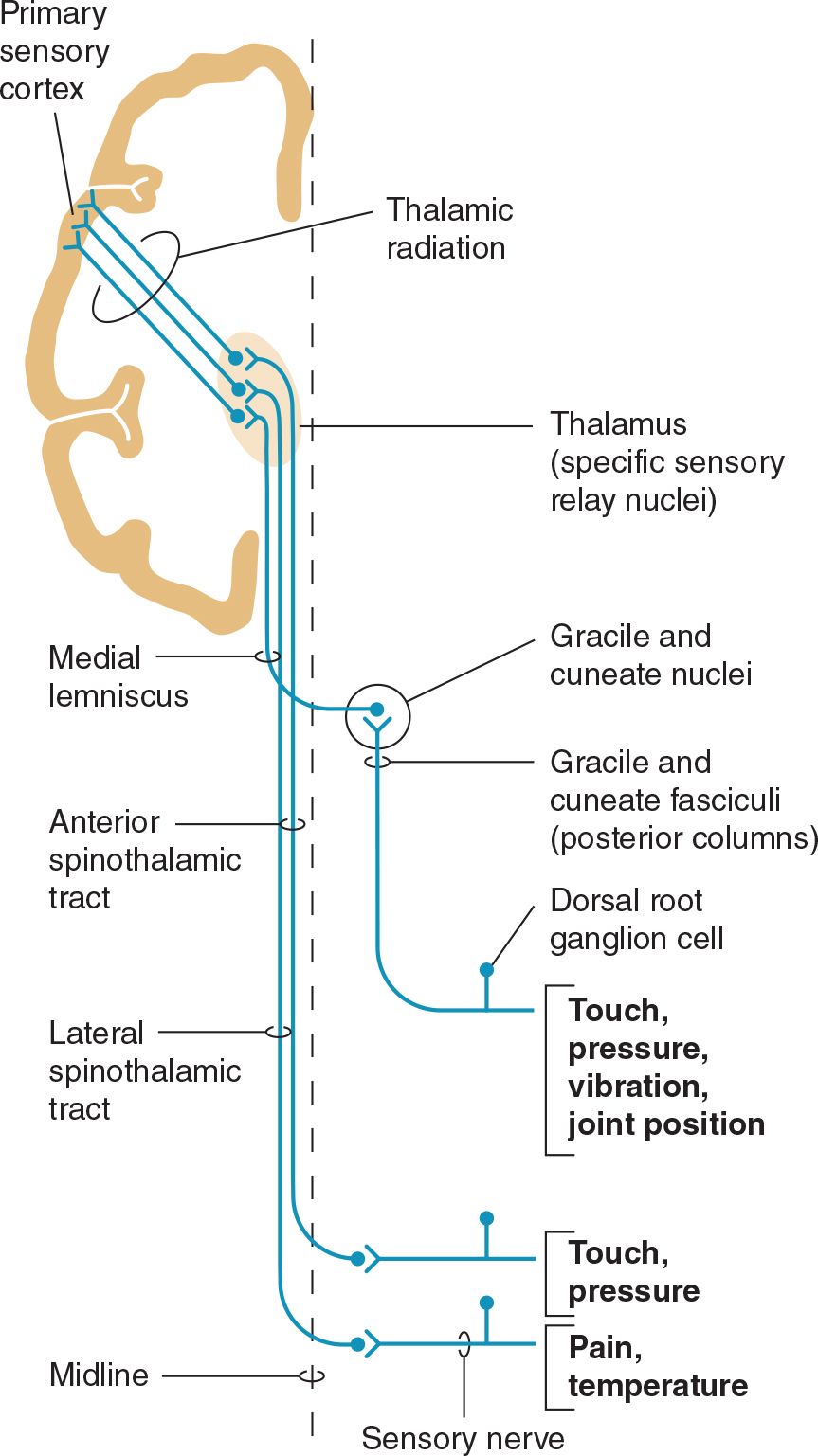
![]() Figure 10-1. Sensory pathways conveying touch, pressure, vibration, joint position, pain, and temperature sensation. (Used with permission from Barrett KE, Barman SM, Boitano S, Brooks H. Ganong’s Review of Medical Physiology. 23rd ed. New York, NY: McGraw-Hill; 2010.)
Figure 10-1. Sensory pathways conveying touch, pressure, vibration, joint position, pain, and temperature sensation. (Used with permission from Barrett KE, Barman SM, Boitano S, Brooks H. Ganong’s Review of Medical Physiology. 23rd ed. New York, NY: McGraw-Hill; 2010.)
First-order sensory neurons from the limbs and trunk have cell bodies in the dorsal root ganglia. Each of these neurons sends a peripheral process that terminates in a free nerve ending or encapsulated sensory receptor and a central process that enters the spinal cord. Sensory receptors are relatively specialized for particular sensations and, in addition to free nerve endings (pain, itch), include Meissner corpuscles, Merkel corpuscles, and hair cells (touch); Krause end-bulbs (cold); and Ruffini corpuscles (heat). First-order sensory neurons synapse centrally at a site that depends on the type of sensation. Fibers mediating touch, pressure, or postural sensation in the limbs and trunk ascend in the posterior columns of the spinal cord to the medulla, where they synapse in the gracile and cuneate nuclei. Other fibers that mediate touch and those subserving pain, temperature, and itch appreciation in the limbs and trunk synapse on neurons in the posterior horns of the spinal cord, particularly in the substantia gelatinosa. First-order sensory neurons from the face, which have cell bodies in the trigeminal (gasserian) ganglion, travel in the trigeminal (V) nerve and enter the pons. Fibers mediating facial touch and pressure synapse in the main trigeminal (V) nerve sensory nucleus, whereas those conveying facial pain and temperature synapse in the spinal trigeminal (V) nerve nucleus.
Second-order sensory neurons with cell bodies in the gracile and cuneate nuclei cross the midline and ascend in the medial lemniscus. Second-order sensory neurons that arise in the posterior horns of the spinal cord cross the midline and ascend in the anterolateral part of the cord: fibers mediating touch pass upward in the anterior spinothalamic tract, whereas pain, itch, and temperature fibers generally travel in the lateral spinothalamic tract. Second-order sensory neurons from the limbs and trunk are joined in the brainstem by fibers from the face: those that mediate facial touch and pressure sensation project from the main trigeminal (V) nerve sensory nucleus via the trigeminal lemniscus, and those that convey facial pain, itch, and temperature project from the spinal trigeminal (V) nerve nucleus via the trigeminothalamic tract, to the ipsilateral thalamus. In the thalamus, medial lemniscal and spinothalamic fibers synapse in the ventral posterolateral (VPL) nucleus; spinothalamic fibers also synapse in the ventral posteroinferior (VPI) nucleus and intralaminar (ILa) nuclei; and fibers in the trigeminal lemniscus and trigeminothalamic tract synapse in the ventral posteromedial (VPM) nucleus. In addition, some second-order spinothalamic sensory neurons send collaterals to the reticular formation.
Third-order sensory neurons project from the thalamus to ipsilateral cerebral cortex. Fibers from VPL, VPI, and VPM travel primarily to primary somatosensory cortex in the postcentral gyrus; fibers from ILa also project to striatum, cingulate gyrus, and prefrontal cortex.
HISTORY
Sensory disturbances may consist of loss of sensation, abnormal sensations, or pain. The term paresthesia denotes abnormal spontaneous sensations, such as burning, tingling, or pins and needles, whereas dysesthesia denotes any unpleasant sensation produced by a stimulus that is normally painless. The term numbness is often used by patients to describe a sense of heaviness, weakness, or deadness in part of the body—and sometimes to signify any sensory impairment; the meaning must be clarified whenever this word is used.
It is important to determine the location of sensory symptoms; their mode of onset and progression; whether they are constant or episodic in nature; whether any factors specifically produce, enhance, or relieve them; and whether there are any accompanying symptoms.
The location of symptoms may reflect their origin. For example, sensory disturbances involving all the limbs suggest peripheral neuropathy, a cervical cord or brainstem lesion, or a metabolic disturbance such as hyperventilation syndrome. Involvement of one entire limb, or of one side of the body, suggests a central (brain or spinal cord) lesion. A hemispheric or brainstem lesion may cause lateralized sensory symptoms, but the face is also commonly affected. In addition, there may be other symptoms and signs, such as aphasia, apraxia, and visual field defects with hemispheric disease, or dysarthria, weakness, vertigo, diplopia, disequilibrium, and ataxia with brainstem disorders. Involvement of part of a limb or a discrete region of the trunk raises the possibility of a nerve or root lesion, depending on the precise distribution. With a root lesion, symptoms may show some relationship to neck or back movements, and pain is often conspicuous.
The course of sensory complaints provides a guide to their cause. Intermittent or repetitive transient symptoms may represent sensory seizures, ischemic phenomena, or metabolic disturbances such as those accompanying hyperventilation. Intermittent localized symptoms that occur at a consistent time may suggest the diagnosis or an exogenous precipitating factor. For example, the pain and paresthesias of carpal tunnel syndrome (median nerve compression at the wrist) characteristically occur at night and awaken the patient from sleep.
SENSORY EXAMINATION
Various sensory modalities are tested in turn, and the distribution of any abnormality is plotted with particular reference to the normal root and peripheral nerve territories. Complete loss of touch appreciation is anesthesia, partial loss is hypesthesia, and increased sensitivity is hyperesthesia. The corresponding terms for pain appreciation are analgesia, hypalgesia, and hyperalgesia or hyperpathia; allodynia refers to the misperception of a trivial tactile sensation as pain.
PRIMARY SENSORY MODALITIES
 Light Touch
Light Touch
The appreciation of light touch is evaluated with a wisp of cotton wool that is brought down carefully on a small region of skin. The patient lies with eyes closed, and indicates each time the stimulus is felt. The appreciation of light touch depends on fibers that traverse the posterior column of the spinal cord in the gracile (leg) and cuneate (arm) fasciculi ipsilaterally (Figures 10-1 and 10-2), passing to the medial lemniscus of the brainstem (Figure 10-3), and on fibers in the contralateral anterior spinothalamic tract.
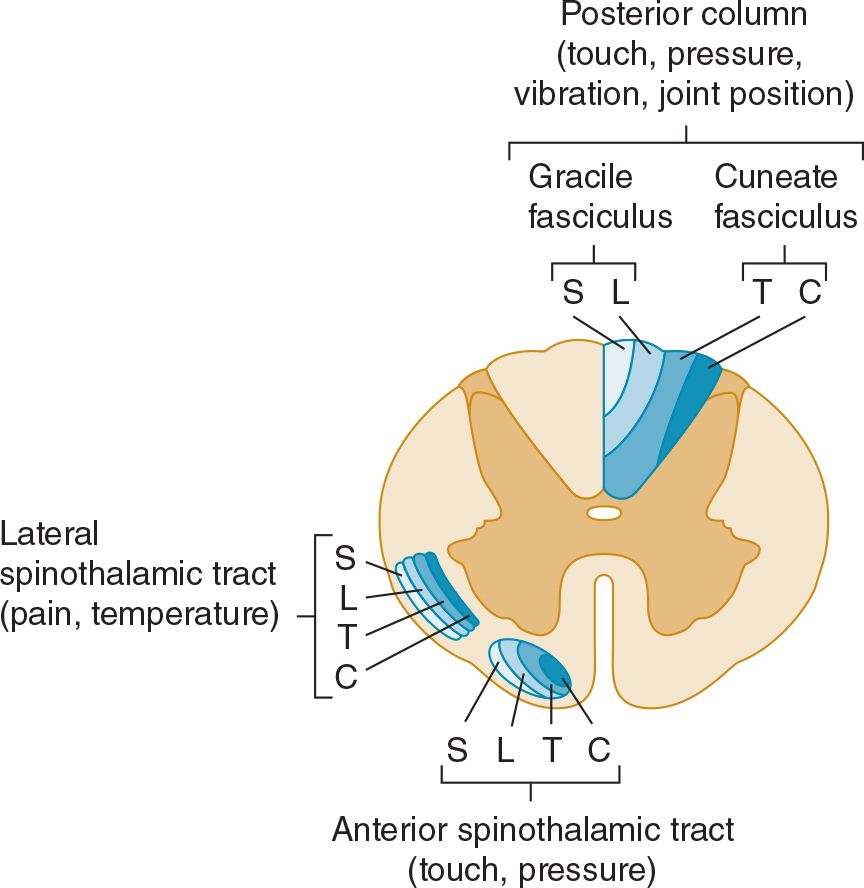
![]() Figure 10-2. Location and lamination of sensory pathways in the spinal cord. C (cervical), T (thoracic), L (lumbar), and S (sacral) indicate the level of origin of fibers within each tract.
Figure 10-2. Location and lamination of sensory pathways in the spinal cord. C (cervical), T (thoracic), L (lumbar), and S (sacral) indicate the level of origin of fibers within each tract.
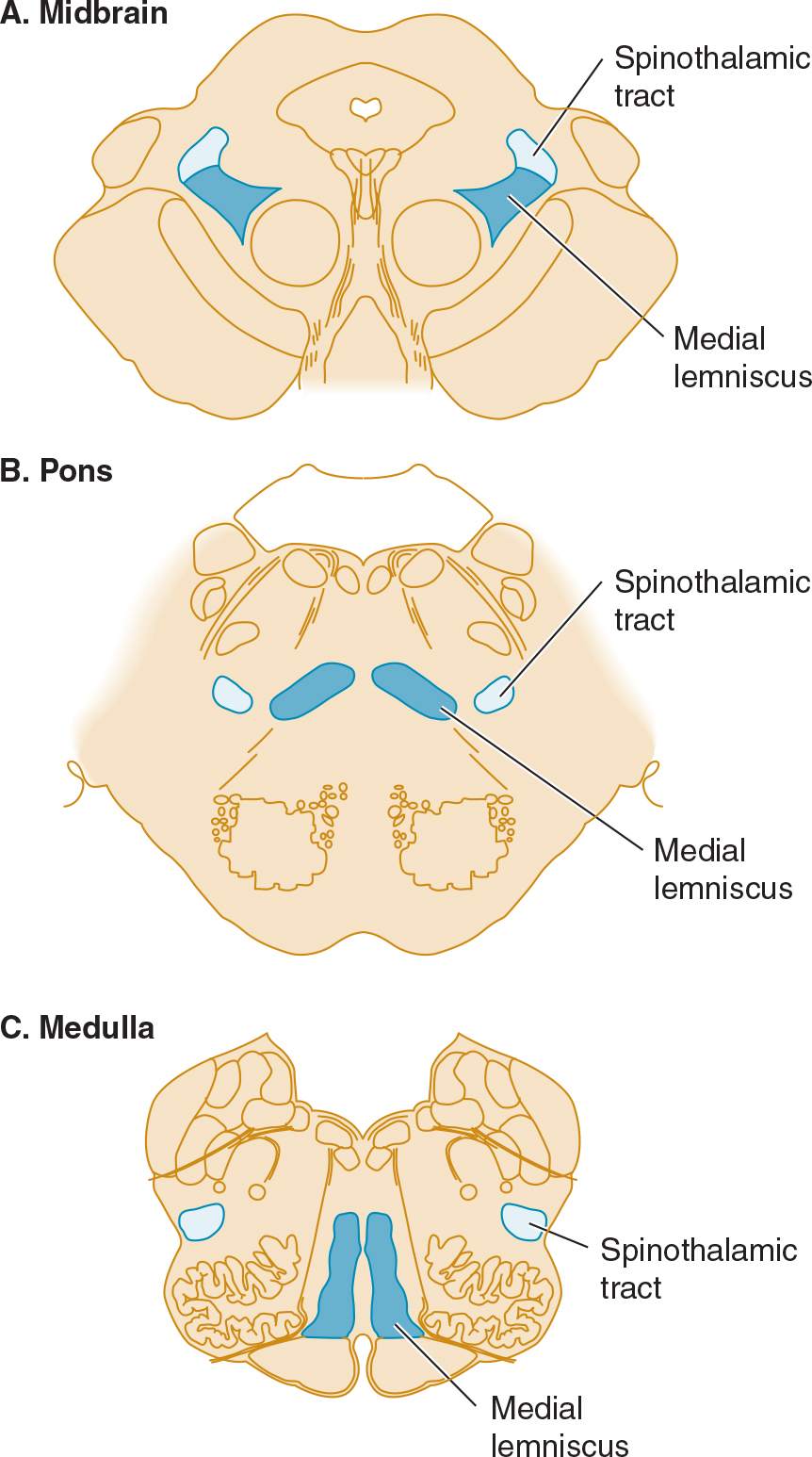
![]() Figure 10-3. Sensory pathways in the brainstem. In the medulla, spinothalamic fibers conveying pain and temperature sensation are widely separated from medial lemniscal fibers mediating touch and pressure; these pathways converge as they ascend in the pons and midbrain.
Figure 10-3. Sensory pathways in the brainstem. In the medulla, spinothalamic fibers conveying pain and temperature sensation are widely separated from medial lemniscal fibers mediating touch and pressure; these pathways converge as they ascend in the pons and midbrain.
 Pinprick & Temperature
Pinprick & Temperature
Pinprick appreciation is tested by asking the patient to indicate whether the point of a pin (not a hypodermic needle, which may puncture the skin and draw blood) feels sharp or blunt. Appreciation of sharpness must be distinguished from appreciation of pressure or touch. The pin—a potential source of infection—should be handled with care and discarded after use. Temperature appreciation is evaluated by applying containers of hot or cold water to the skin. For convenience, cold can be tested by application of the side of a metal tuning fork to different bodily regions; testing of heat is usually omitted from the routine examination. Pinprick and temperature appreciation depend on the integrity of the lateral spinothalamic tracts (see Figures 10-1 and 10-2). The afferent fibers cross in front of the central canal after ascending for two or three segments from their level of entry into the cord.
 Deep Pressure
Deep Pressure
Deep pressure sensibility is evaluated by pressure on the tendons, such as the Achilles tendon at the ankle.
 Vibration
Vibration
A tuning fork (128 Hz) is set in motion and then placed over a bony prominence; the patient is asked to indicate whether vibration, rather than simple pressure, is felt. Many healthy elderly patients have impaired appreciation of vibration below the knees.
 Joint Position
Joint Position
The patient is asked to indicate the direction of small passive movements of the terminal interphalangeal joints of the fingers and toes. Patients with severe impairment of joint position sense may exhibit slow, continuous movement of the fingers (pseudoathetoid movement) when attempting to hold the hands outstretched with eyes closed. For clinical purposes, both joint position sense and the ability to appreciate vibration are considered to depend on fibers carried in the posterior columns of the spinal cord, although this is not strictly true for vibration.
COMPLEX SENSORY FUNCTIONS
 Romberg Test
Romberg Test
The patient assumes a steady stance with feet together, arms outstretched, and eyes closed and is observed for any tendency to sway or fall. The test is positive (abnormal) if unsteadiness is markedly increased by eye closure—as occurs, for example, in tabes dorsalis. A positive test is indicative of grossly impaired joint position sense in the legs.
 Two-Point Discrimination
Two-Point Discrimination
The ability to distinguish simultaneous touch at two neighboring points depends on the integrity of the central and peripheral nervous system, the degree of separation of the two points, and the part of the body that is stimulated. The patient indicates whether he or she is touched by one or two compass points, while the distance between the points is varied in order to determine the shortest distance at which they are recognized as different points. The threshold for two-point discrimination approximates 4 mm at the fingertips and may be several centimeters on the back. When peripheral sensory function is intact, impaired two-point discrimination suggests a disorder affecting the sensory cortex.
 Graphesthesia, Stereognosis, & Barognosis
Graphesthesia, Stereognosis, & Barognosis
Agraphesthesia, the inability to identify a number traced on the skin of the palm of the hand despite normal cutaneous sensation, implies a lesion involving the contralateral parietal lobe. The same is true of inability to distinguish between various shapes or textures by touch (astereognosis) or impaired ability to distinguish between different weights (abarognosis).
 Bilateral Sensory Discrimination
Bilateral Sensory Discrimination
In some patients with apparently normal sensation, simultaneous stimulation of the two sides of the body reveals an apparent neglect of (or inattention to) sensation from one side, usually because of an underlying contralateral cerebral lesion.
SENSORY CHANGES & THEIR SIGNIFICANCE
The nature and distribution of any sensory change must be determined. Failure to find clinical evidence of sensory loss in patients with sensory symptoms should not imply that the symptoms necessarily have a psychogenic basis. Sensory symptoms often develop well before the onset of sensory signs.
PERIPHERAL NERVE LESIONS
 Mononeuropathy
Mononeuropathy
In patients with a lesion of a single peripheral nerve, sensory loss is usually less than predicted on anatomic grounds because of overlap from adjacent nerves. Moreover, depending on the type of lesion, the fibers in a sensory nerve may be affected differently. Compressive lesions, for example, tend to affect preferentially the large fibers subserving touch.
 Polyneuropathy
Polyneuropathy
In patients with polyneuropathies, sensory loss is generally symmetric and is greater distally than proximally (stocking-and-glove sensory loss or length-dependent neuropathy). The loss generally will have progressed almost to the knees before the hands are affected. Sensory loss may then be accompanied by a motor deficit and reflex changes. Diabetes mellitus, amyloidosis, and certain other metabolic disorders (eg, Tangier disease, a recessive trait characterized by the near absence of high-density lipoproteins) preferentially involve small nerve fibers subserving pain and temperature appreciation; in a pure small-fiber sensory neuropathy, the tendon reflexes are unaffected and no motor deficit occurs.
ROOT LESIONS
Nerve root involvement produces impaired cutaneous sensation in a segmental pattern (Figure 10-4), but because of overlap there is generally no sensory loss unless two or more adjacent roots are affected. Pain is often a conspicuous feature with compressive root lesions. Depending on the level affected, there may be loss of tendon reflexes (C5-C6, biceps and brachioradialis; C7-C8, triceps; L3-L4, knee; S1, ankle), and if the anterior roots are also involved, weakness and muscle atrophy may occur.
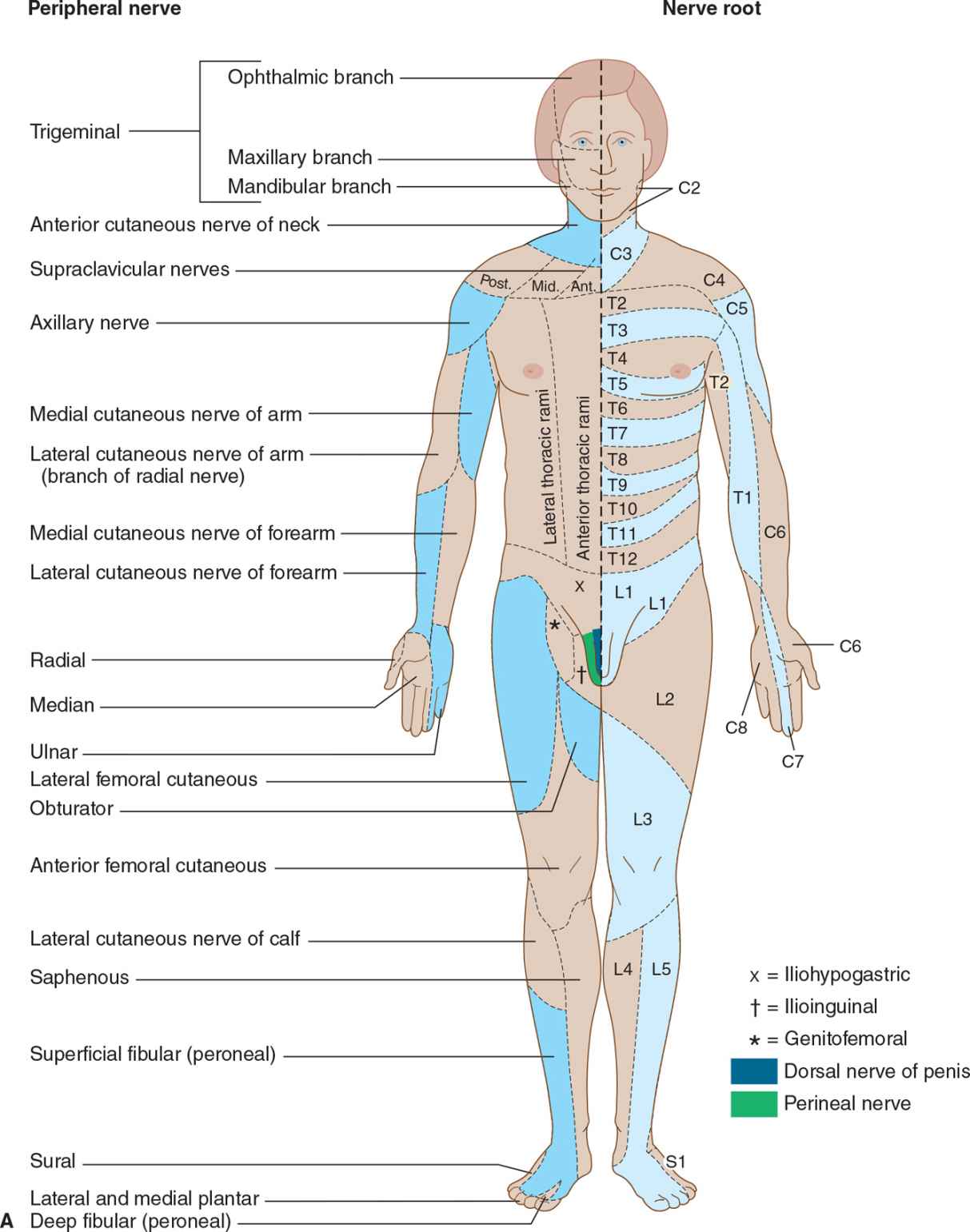
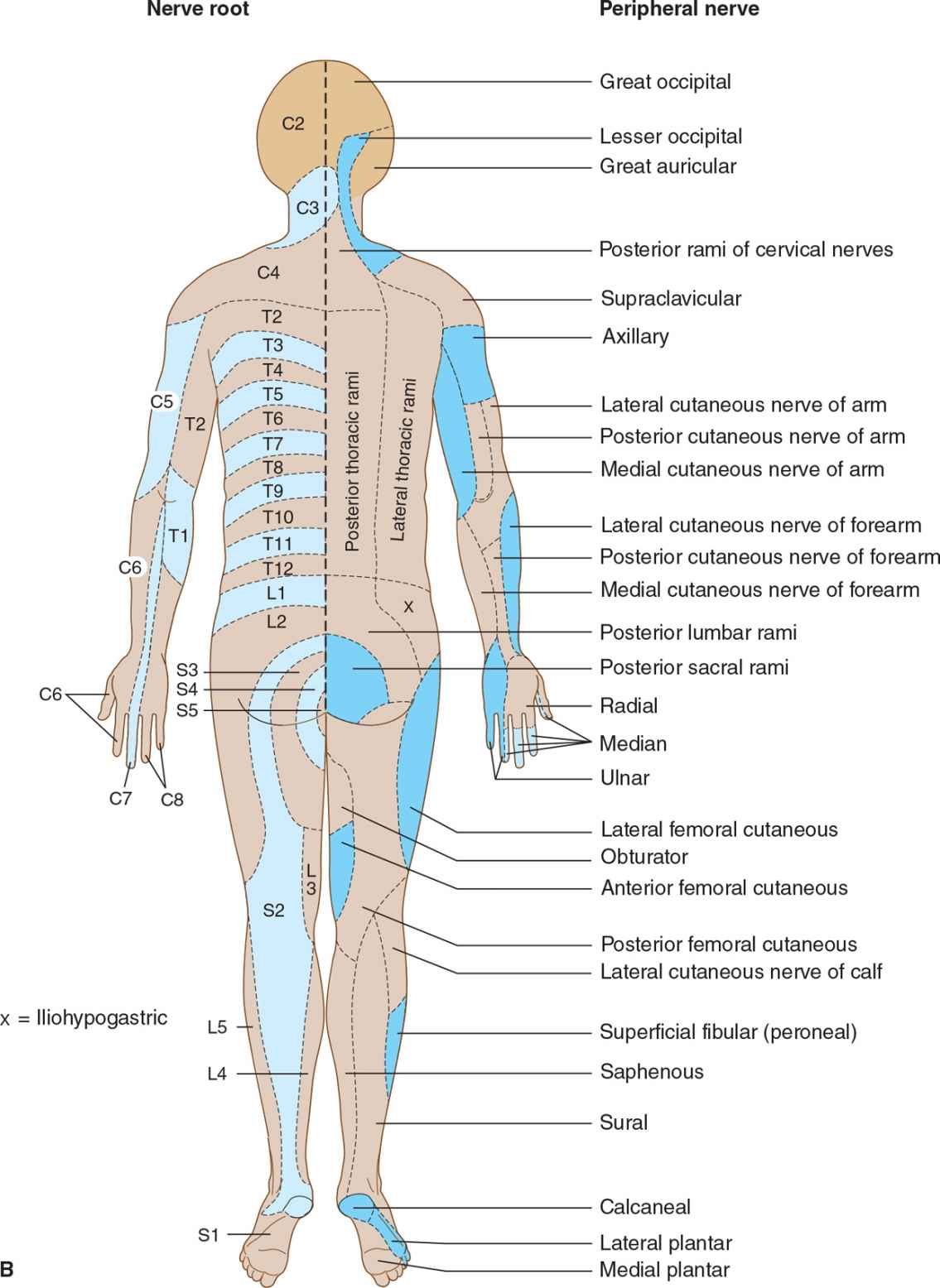
![]() Figure 10-4. (A) Cutaneous innervation (anterior view). The segmental or radicular (nerve root) distribution is shown on the left side of the body, and the peripheral nerve distribution on the right side of the body. (B) Cutaneous innervation (posterior view). The segmental or radicular (nerve root) distribution is shown on the left side of the body, and the peripheral nerve distribution on the right side of the body. For details of radial, median, ulnar, fibular (peroneal), and femoral nerves, see Appendix.
Figure 10-4. (A) Cutaneous innervation (anterior view). The segmental or radicular (nerve root) distribution is shown on the left side of the body, and the peripheral nerve distribution on the right side of the body. (B) Cutaneous innervation (posterior view). The segmental or radicular (nerve root) distribution is shown on the left side of the body, and the peripheral nerve distribution on the right side of the body. For details of radial, median, ulnar, fibular (peroneal), and femoral nerves, see Appendix.
SPINAL CORD LESIONS
In patients with spinal cord lesions, there may be a transverse sensory level. Physiologic areas of increased sensitivity do occur, however, at the costal margin, over the breasts, and in the groin, and these must not be taken as abnormal. Therefore, the level of a sensory deficit affecting the trunk is best determined by careful sensory testing over the back rather than the chest and abdomen.
 Central Cord Lesion
Central Cord Lesion
With a central cord lesion, such as occurs in syringomyelia, after trauma, and with certain tumors, there is characteristically a loss of pain and temperature appreciation with sparing of other modalities. This loss is due to the interruption of fibers conveying pain and temperature that cross from one side of the cord to the spinothalamic tract on the other. Such a loss is usually bilateral, may be asymmetric, and involves only the fibers of the involved segments. It may be accompanied by lower motor neuron weakness in the muscles supplied by the affected segments and sometimes by a pyramidal and posterior column deficit below the lesion (Figure 10-5).
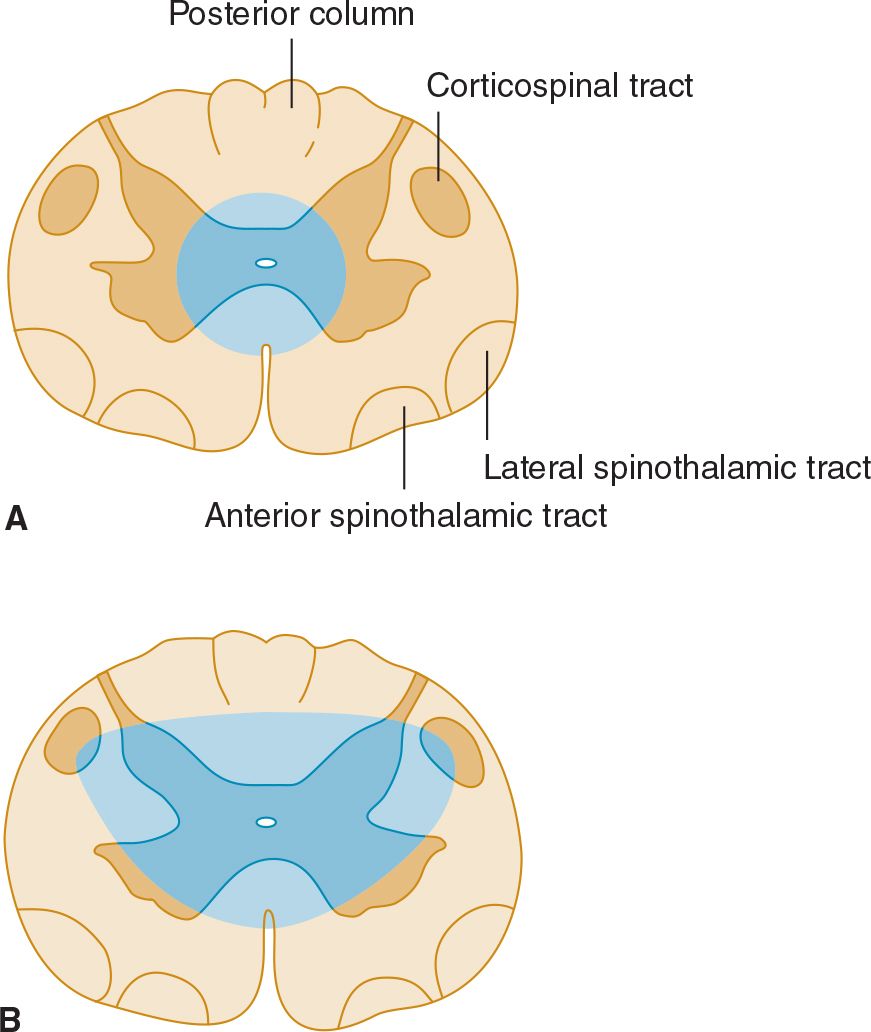
![]() Figure 10-5. Central cord lesions (blue) of moderate (A) or marked (B) extent. Less extensive lesions impair pain and temperature appreciation by interrupting incoming sensory fibers as they cross to the contralateral spinothalamic tract; involvement of anterior horn cells causes lower motor neuron weakness. These deficits are restricted to dermatomes and muscles innervated by the involved spinal cord segments. More extensive lesions also produce disturbances of touch, pressure, vibration, and joint position sense because of involvement of the posterior columns and cause pyramidal signs because of corticospinal tract involvement, especially affecting the arms (see lamination of corticospinal tract in Figure 9-6). These deficits occur below the level of the lesion.
Figure 10-5. Central cord lesions (blue) of moderate (A) or marked (B) extent. Less extensive lesions impair pain and temperature appreciation by interrupting incoming sensory fibers as they cross to the contralateral spinothalamic tract; involvement of anterior horn cells causes lower motor neuron weakness. These deficits are restricted to dermatomes and muscles innervated by the involved spinal cord segments. More extensive lesions also produce disturbances of touch, pressure, vibration, and joint position sense because of involvement of the posterior columns and cause pyramidal signs because of corticospinal tract involvement, especially affecting the arms (see lamination of corticospinal tract in Figure 9-6). These deficits occur below the level of the lesion.
 Anterolateral Spinal Cord Lesion
Anterolateral Spinal Cord Lesion
Lesions involving the anterolateral portion of the spinal cord (lateral spinothalamic tract) can cause contralateral impairment of pain and temperature appreciation in segments below the level of the lesion. The spinothalamic tract is laminated, with fibers from the sacral segments the outermost. Intrinsic cord (intramedullary) lesions often spare the sacral fibers, whereas extramedullary lesions, which compress the cord, tend to involve these fibers as well as those arising from more rostral levels.
 Anterior Spinal Cord Lesion
Anterior Spinal Cord Lesion
With destructive lesions involving predominantly the anterior portion of the spinal cord, pain and temperature appreciation are impaired below the level of the lesion from lateral spinothalamic tract involvement. In addition, weakness or paralysis of muscles supplied by the involved segments of the cord results from damage to anterior horn cells. With more extensive disease, involvement of the corticospinal tracts in the lateral funiculi may cause a pyramidal deficit below the lesion. There is relative preservation of posterior column function (Figure 10-6). Ischemic myelopathies caused by occlusion of the anterior spinal artery take the form of anterior spinal cord lesions.
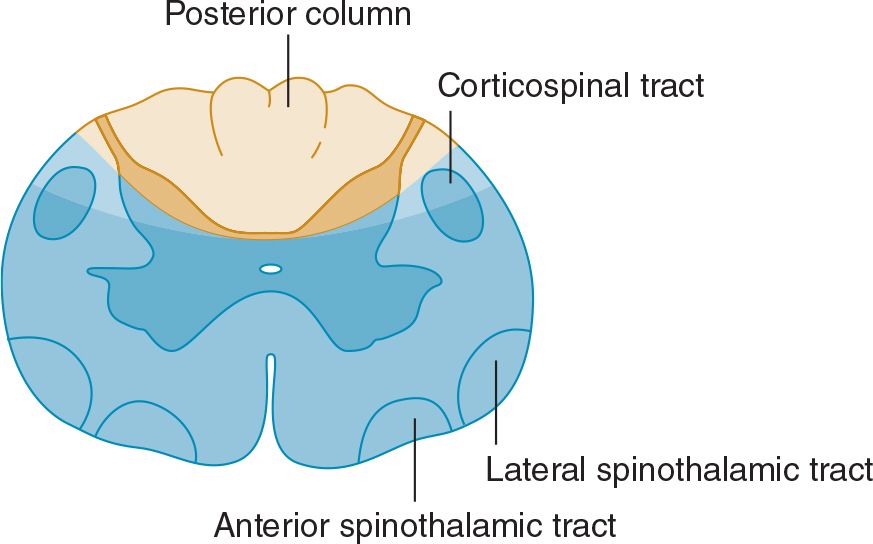
![]() Figure 10-6. Anterior spinal cord lesion (blue) associated with occlusion of the anterior spinal artery. Clinical features are similar to those seen with severe central cord lesions (see Figure 10-5B), except that posterior column sensory functions are spared and the defect in pain and temperature sensation extends to sacral levels.
Figure 10-6. Anterior spinal cord lesion (blue) associated with occlusion of the anterior spinal artery. Clinical features are similar to those seen with severe central cord lesions (see Figure 10-5B), except that posterior column sensory functions are spared and the defect in pain and temperature sensation extends to sacral levels.
 Posterior Column Lesion
Posterior Column Lesion
A patient with a posterior column lesion may complain of a tight or bandlike sensation in the regions corresponding to the level of spinal involvement and sometimes also of paresthesias (like electric shocks) radiating down the extremities on neck flexion (Lhermitte sign). There is loss of vibration and joint position sense below the level of the lesion, with preservation of other sensory modalities. The deficit may resemble that resulting from involvement of large fibers in the posterior roots.
 Spinal Cord Hemisection
Spinal Cord Hemisection
Lateral hemisection of the spinal cord leads to Brown-Séquard syndrome. Below the lesion, there is an ipsilateral pyramidal deficit and disturbed appreciation of vibration and joint position sense, and contralateral loss of pain and temperature appreciation that begins two or three segments below the lesion (Figure 10-7). Hyperalgesia and spontaneous pain are sometimes prominent ipsilaterally.
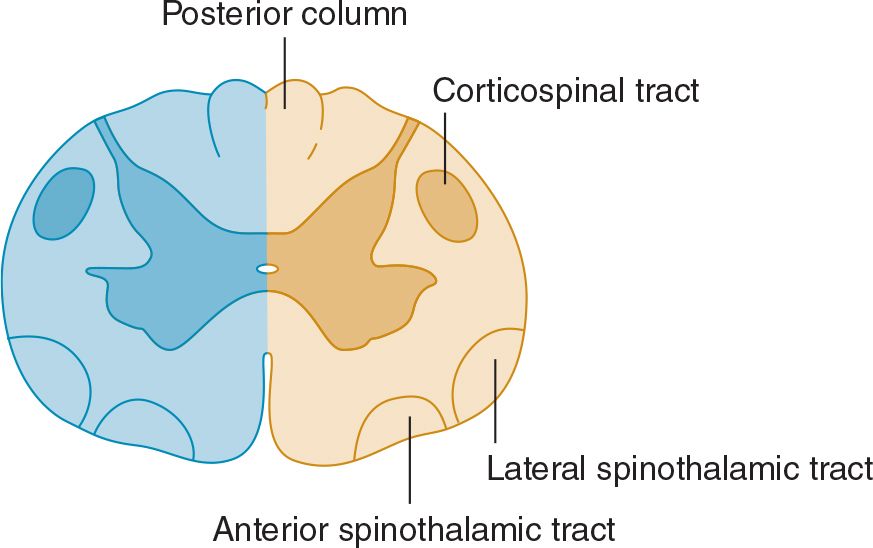
![]() Figure 10-7. Spinal cord lesion (blue) in Brown-Séquard syndrome. Lateral hemisection of the spinal cord causes ipsilateral pyramidal dysfunction and impairment of posterior column sensory function below the level of the lesion, and contralateral impairment of pain and temperature sensation with an upper limit slightly below the level of the lesion.
Figure 10-7. Spinal cord lesion (blue) in Brown-Séquard syndrome. Lateral hemisection of the spinal cord causes ipsilateral pyramidal dysfunction and impairment of posterior column sensory function below the level of the lesion, and contralateral impairment of pain and temperature sensation with an upper limit slightly below the level of the lesion.
BRAINSTEM LESIONS
With brainstem lesions, sensory disturbances may be accompanied by a motor deficit, cerebellar signs, and cranial nerve palsies.
With lesions involving the spinothalamic tract in the dorsolateral medulla and pons, pain and temperature appreciation are lost in the limbs and trunk on the opposite side of the body. When such a lesion is medullary, it also typically involves the spinal trigeminal nucleus, impairing pain and temperature sensation on the same side of the face as the lesion. The result is a crossed sensory deficit that affects the ipsilateral face and contralateral limbs. In contrast, spinothalamic lesions above the spinal trigeminal nucleus affect the face, limbs, and trunk contralateral to the lesion. With lesions affecting the medial lemniscus, there is loss of touch and proprioception on the opposite side of the body. In the upper brainstem, the spinothalamic tract and medial lemniscus run together so that a single lesion may cause loss of all superficial and deep sensation over the contralateral side of the body (see Figure 10-3).
THALAMIC LESIONS
Thalamic lesions may lead to loss or impairment of all forms of sensation on the contralateral side of the body, and this may have a distribution that differs from the area of symptomatic involvement. Spontaneous pain, sometimes with a particularly unpleasant quality, may occur on the affected side. Patients may describe it as burning, tearing, knifelike, or stabbing, but often have difficulty characterizing it. Any form of cutaneous stimulation can lead to painful or unpleasant sensations. Such a thalamic syndrome (Dejerine-Roussy syndrome) can also occasionally result from lesions of the white matter of the parietal lobe or from spinal cord lesions, as discussed later.
LESIONS OF THE SENSORY CORTEX
Disease limited to the sensory cortex impairs discriminative sensory function on the opposite side of the body. Patients may be unable to localize stimuli on the affected side or to recognize the position of different parts of the body. They may not be able to recognize objects by touch or to estimate their size, weight, consistency, or texture. Cortical sensory disturbances are usually more conspicuous in the hands than in the trunk or proximal portions of the limbs.
DISTINCTION BETWEEN ORGANIC & PSYCHOGENIC SENSORY DISTURBANCES
Psychogenic disturbances of sensation may be associated with such psychiatric disturbances as conversion disorder. They may take any form but most often are restricted to loss of cutaneous sensation. There may be several characteristic features.
Nonorganic sensory loss does not conform in its distribution to any specific neuroanatomic pattern. It may surround a bony landmark or involve an area defined by surface landmarks rather than innervation. Indeed, it is not uncommon for there to be an apparent loss of sensation in one or more extremities, with circumferential margins in the axilla or groin; organic sensory loss with such a margin is unusual. Organic peripheral sensory loss over the trunk or face does not usually extend to the midline but stops 3 to 5 cm before it, because of overlap in the innervation on the two sides; with nonorganic disturbances, apparent sensory loss commonly stops precisely at the midline.
There is often a sudden transition between areas of nonorganic sensory loss and areas with normal sensation. By contrast, with organic disturbances, there is usually an area of altered sensation between insensitive areas and adjacent areas with normal sensibility.
In nonorganic disturbances, there may be a dissociated loss that is difficult to interpret on an anatomic basis. For example, there may be a total loss of pinprick appreciation but preserved temperature sensation. Moreover, despite the apparent loss of posterior column function, the patient may be able to walk normally or maintain the arms outstretched without difficulty or pseudoathetoid movements.
In nonorganic sensory disturbances, appreciation of vibration may be impaired on one side but not the other side of a bony midline structure, such as the skull or sternum. The vibrations are in fact conducted to both sides by the bone, so that even if there is a hemisensory disturbance, the vibrations are appreciated on either side in patients with organic sensory disorders.
Finally, it should be noted that sensory disturbances are often suggested to the patient by the examiner’s own expectations. Such findings can be particularly misleading because they may be neuroanatomically correct. One helpful approach is to have the patient outline on the body the extent of any perceived sensory disturbance before formal sensory testing is undertaken.
PERIPHERAL NERVE LESIONS
Sensory symptoms are usually a conspicuous feature in patients with peripheral nerve lesions (Table 10-1). Sensory impairment may be in a distal stocking-and-glove pattern in patients with polyneuropathies or may follow the pattern of individual peripheral nerves in patients with mononeuropathies (see Figure 10-4).
Table 10-1. Causes of Peripheral Neuropathy.
Idiopathic inflammatory neuropathies
Acute idiopathic polyneuropathy (Guillain-Barré syndrome)
Chronic inflammatory demyelinating polyneuropathy
Metabolic and nutritional neuropathies
Diabetes
Other endocrinopathies
Hypothyroidism
Acromegaly
Uremia
Liver disease
Vitamin B12 deficiency
Infective and granulomatous neuropathies
AIDS
Leprosy
Diphtheria
Sarcoidosis
Sepsis and multiorgan failure
Vasculitic neuropathies
Systemic necrotizing vasculitis
Granulomatosis with polyangiitis (Wegener granulomatosis)
Giant cell arteritis
Rheumatoid arthritis
Systemic lupus erythematosus
Sjögren syndrome
Scleroderma
Mixed connective tissue disease
Neoplastic and paraproteinemic neuropathies
Compression and infiltration by tumor
Paraneoplastic syndromes
Paraproteinemias
Amyloidosis
Drug-induced and toxic neuropathies
Alcohol
Therapeutic drugs (see Table 10-2)
Toxins
Organic compounds
Hexacarbons
Organophosphates
Heavy metals
Arsenic
Lead
Thallium
Gold
Platinum
Tryptophan (contaminant)
Hereditary neuropathies
Idiopathic
Hereditary motor and sensory neuropathies
Hereditary sensory and autonomic neuropathies
Familial amyloidosis
Friedreich ataxia
Hereditary neuropathy with liability to pressure palsies
Metabolic
Porphyria
Metachromatic leukodystrophy
Krabbe disease
Abetalipoproteinemia
Tangier disease
Refsum disease
Fabry disease
Entrapment neuropathies
CLASSIFICATION
MONONEUROPATHY SIMPLEX
This term signifies involvement of a single peripheral nerve.
MONONEUROPATHY MULTIPLEX
In this disorder, several individual nerves are affected, usually at random and noncontiguously. Clinical examination reveals a clinical deficit attributable to involvement of one or more isolated peripheral nerves, except when mononeuropathy multiplex is extensive and the resulting deficits become confluent.
POLYNEUROPATHY
The term polyneuropathy denotes a disorder in which the function of several peripheral nerves is affected at the same time. This leads to a predominantly distal and symmetric deficit, with loss of tendon reflexes except when small fibers are selectively involved. Polyneuropathies are sometimes subclassified according to the primary site at which the nerve is affected.
In distal axonopathies (axonal neuropathies), the axon is the principal pathologic target; most polyneuropathies fall into this category.
Myelinopathies (demyelinating neuropathies) are conditions that involve the myelin sheath surrounding the axon. These disorders include acute idiopathic polyneuropathy (Guillain-Barré syndrome), chronic inflammatory demyelinating neuropathy, diphtheria, certain paraneoplastic and paraproteinemic states, and various hereditary conditions including metachromatic leukodystrophy, Krabbe disease, and types 1 and 3 Charcot-Marie-Tooth hereditary motor and sensory neuropathy (CMT-1 and 3).
Finally, certain disorders, termed neuronopathies, principally affect nerve cell bodies in the anterior horn of the spinal cord or dorsal root ganglion. Examples are type 2 CMT hereditary motor and sensory neuropathy, pyridoxine-induced neuropathy, and some paraneoplastic syndromes.
CLINICAL FINDINGS
SENSORY DISTURBANCES
Involvement of sensory fibers can lead to numbness and impaired sensation; abnormal spontaneous sensations, such as pain and paresthesias; and perverted sensations such as hyperpathia (amplified response to a normally painful stimulus).
 Pain
Pain
Pain is a conspicuous feature of certain neuropathies, especially if small fibers within the nerves are affected. The precise mechanism of its genesis is unclear. Polyneuropathies associated with prominent pain include those related to diabetes, alcoholism, porphyria, Fabry disease, amyloidosis, rheumatoid arthritis, and acquired immunodeficiency syndrome (AIDS), as well as dominantly inherited sensory neuropathy and paraneoplastic sensory neuronopathy. Pain is also a feature of many entrapment neuropathies and of idiopathic brachial plexopathy.
 Dissociated Sensory Loss
Dissociated Sensory Loss
Dissociated sensory loss is impairment of some sensory modalities, such as pain and temperature, with preservation of others, such as light touch, vibration, and joint position sense. Although the presence of a dissociated sensory loss often indicates a spinal cord lesion, it also occurs in peripheral neuropathies when there is selective involvement of peripheral nerve fibers of a certain size, such as occurs in amyloid neuropathy, leprous neuritis, or hereditary sensory neuropathy. Small fiber disease is commonly associated with disproportionate impairment of pain and temperature appreciation, spontaneous pain, and autonomic dysfunction. Large fiber disease, by contrast, results in defective touch, vibration, and joint position sense, early loss of tendon reflexes, and prominent motor symptoms.
MOTOR DEFICITS
Peripheral nerve lesions may cause weakness of muscles innervated by the nerve, accompanied in severe cases by wasting and fasciculation. There may be difficulty in the performance of fine tasks; this is compounded by any accompanying sensory loss. The clinical findings reflect a lower motor neuron deficit, and it is the distribution of these signs and the presence of accompanying sensory and reflex changes that suggest they may be due to peripheral nerve involvement.
TENDON REFLEXES
These are impaired or lost if reflex arcs are interrupted on either the afferent or efferent side (C5-C6, biceps and brachioradialis; C7-C8, triceps; L3-L4, knee; S1, ankle). The ankle reflexes are usually the first to be lost in patients with polyneuropathies, but may also be absent in healthy elderly subjects.
AUTONOMIC DISTURBANCES
Autonomic disturbances are particularly conspicuous in some peripheral neuropathies—especially Guillain-Barré syndrome and neuropathies related to diabetes, renal failure, porphyria, certain paraneoplastic disorders, and amyloidosis. Symptoms include postural hypotension, tachycardia, cold extremities, impaired thermoregulatory sweating, bladder and bowel dysfunction, and impotence.
ENLARGED NERVES
Palpably enlarged peripheral nerves raise the possibility of leprosy, amyloidosis, hereditary motor and sensory neuropathies, Refsum disease, acromegaly, or chronic inflammatory demyelinating polyneuropathy.
EVALUATION OF PATIENTS
TIME COURSE
Polyneuropathy that develops acutely over a few days usually relates to an inflammatory process, as in the Guillain-Barré syndrome. It may also relate to an underlying neoplasm, to infections such as diphtheria, to metabolic disorders such as acute intermittent porphyria, or to exposure to such toxic substances as thallium or triorthocresyl phosphate. A chronic course with a gradual evolution over several years is typical of many hereditary or metabolic polyneuropathies but also characterizes chronic inflammatory demyelinating polyneuropathy.
Mononeuropathy of acute onset is likely to be traumatic or ischemic in origin, whereas one evolving gradually is more likely to relate to entrapment (ie, compression by neighboring anatomic structures) or to recurrent minor trauma.
AGE AT ONSET
Polyneuropathy that develops during childhood or early adult life often has a hereditary basis, but may also relate to an underlying inflammatory disorder. Polyneuropathy developing later is more likely to result from a metabolic, toxic, or inflammatory disorder, or from an underlying neoplasm.
Mononeuropathy presenting in the neonatal period is likely to be developmental in origin or related to birth injury; one presenting in later life may relate to entrapment or injury that is often occupationally determined.
OCCUPATIONAL HISTORY
Various industrial toxins can lead to peripheral neuropathy, including carbon disulfide, n-hexane, ethylene oxide, methyl bromide, acrylamide, triorthocresyl phosphate and certain other organophosphates, DDT, arsenic, lead, and thallium. A mononeuropathy is sometimes the first clinical manifestation of an occupationally related polyneuropathy, but it may also develop in response to entrapment or recurrent minor occupational trauma. For example, carpal tunnel syndrome is more common in persons who do heavy manual labor or develop repetitive motion injury as a result of computer terminal use, and a lesion of the deep palmar branch of the ulnar nerve may relate to repeated pressure on the palm of the hand by, for example, punching down heavily on a stapler or using heavy equipment such as a pneumatic road drill.
MEDICAL HISTORY
 Metabolic Disorders
Metabolic Disorders
Peripheral neuropathy may relate to metabolic disorders such as diabetes mellitus, uremia, liver disease, myxedema, acromegaly, metachromatic leukodystrophy, or Fabry disease. That caused by diabetes is especially important and may take the form of an entrapment mononeuropathy, acute ischemic mononeuropathy, distal sensorimotor polyneuropathy, subacute proximal motor polyradiculoplexopathy (diabetic amyotrophy), thoracoabdominal radiculopathy, or autonomic neuropathy.
 Neoplasm
Neoplasm
The peripheral nerves, spinal nerves, and limb plexuses may be compressed or infiltrated by extension of primary tumors or metastatic lymph nodes. Neoplastic disease can also lead to a nonmetastatic (paraneoplastic) sensory or sensorimotor polyneuropathy or to Lambert-Eaton syndrome, a disorder of neuromuscular transmission discussed in Chapter 9, Motor Disorders.
 Connective Tissue Disorders
Connective Tissue Disorders
Polyarteritis nodosa, rheumatoid arthritis, Churg-Strauss syndrome, and granulomatosis with polyangiitis (formerly Wegener granulomatosis) may be associated with mononeuropathy multiplex or, less commonly, polyneuropathy or cranial neuropathy. Polyneuropathy is more common in systemic lupus erythematosus. Rheumatoid arthritis may cause focal entrapment or compressive mononeuropathies in the vicinity of affected joints.
 AIDS
AIDS
Acquired immune deficiency syndrome (AIDS) is commonly associated with a distal, symmetric, primarily sensory polyneuropathy. Less frequently, AIDS is associated with an acute or chronic inflammatory demyelinating polyneuropathy, polyradiculopathy, mononeuropathy multiplex, or autonomic neuropathy. Neuropathies are also seen with asymptomatic human immunodeficiency virus-1 (HIV-1) infection and HIV-1 seroconversion.
DRUG & ALCOHOL HISTORY
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


