FIGURE 36.1 Some common patterns of sensory loss. A. Hemisensory loss due to a hemispheric lesion. B. Crossed sensory loss to pain and temperature due to a lateral medullary lesion. C. Midthoracic spinal cord level. D. Suspended, dissociated sensory loss to pain and temperature due to syringomyelia. E. Distal, symmetric sensory loss due to peripheral neuropathy. F. Crossed spinothalamic loss on one side with posterior column loss on the opposite side due to Brown-Séquard syndrome. G. Dermatomal sensory loss due to cervical radiculopathy. H. Dermatomal sensory loss due to lumbosacral radiculopathy.
Sensory function and motor activity are interdependent, and severe motor disabilities may occur because of impaired sensation. This is particularly evident with parietal lobe lesions, but motor dysfunction may also occur with lesions involving the posterior roots, peripheral nerves posterior columns of the spinal cord, or the other central sensory pathways.
Conversely, motor dysfunction may affect sensory discrimination. When equal weights are placed in a patient’s hands, she may underestimate the weight on the side with cerebellar dysfunction and overestimate it on the side with extrapyramidal dysfunction.
Diminution or perversion of sensation may occur with pathology involving the sensory receptors, but this does not often arise in primary neurologic illnesses. Pain and pruritus due to skin irritation, traumatic denudements, and burns may result from abnormalities of the receptors or the nerve filaments to them, and decreased sensation in callosities and scars may result from involvement of the end-organs and smaller filaments.
In focal peripheral neuropathies, the area of sensory abnormality corresponds to the distribution of the specific involved nerve. The areas of skin supplied by various nerves are shown in Figure 36.2. Within the involved area, all sensory modalities are affected to a greater or lesser degree. Sensory distributions may vary slightly from individual to individual, and the mapped area may not correspond precisely to a published text or atlas. An excellent source for a pictorial/graphic demonstration of peripheral nerve distributions is http://www.neuroguide.com/nerveindex.html. Figure 36.3 demonstrates some of the variability in the cutaneous supply of the superficial radial nerve.
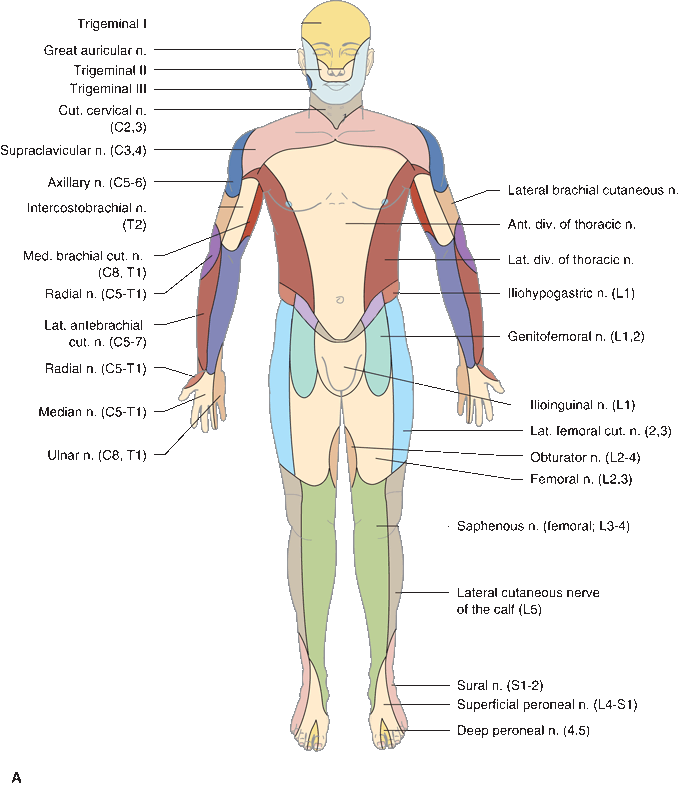
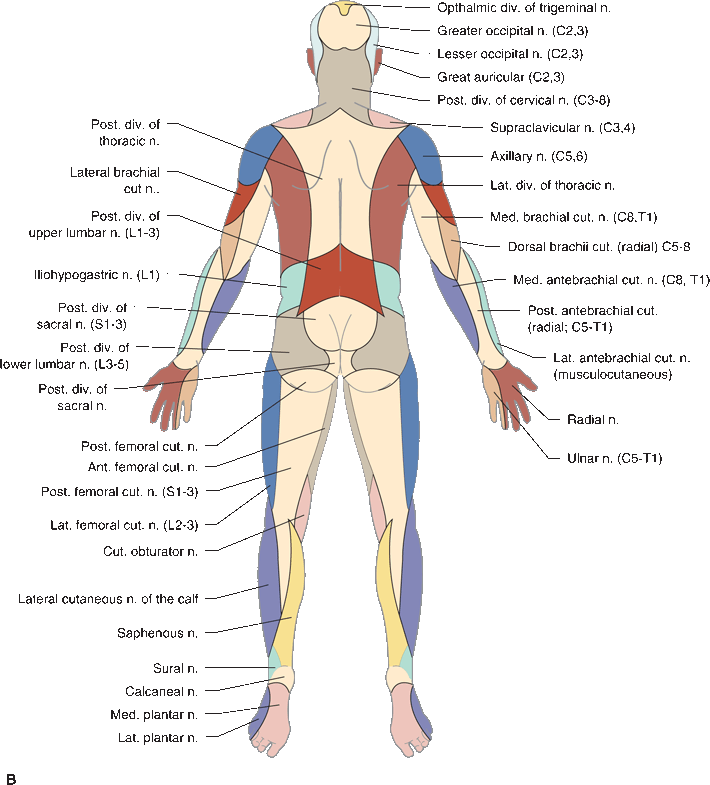
FIGURE 36.2 The cutaneous distribution of the peripheral nerves. A. On the anterior aspect of the body. B. On the posterior aspect of the body.
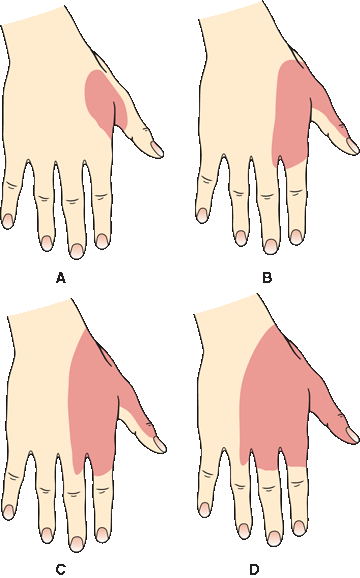
FIGURE 36.3 Variations in the cutaneous distribution of the radial nerve. A. Frequent distribution. B. Typical distribution. C. Frequent distribution. D. Anesthesia beyond the usual limit. (Modified from Tinel J. Nerve Wounds. Rothwell F [trans]. New York, William Wood & Co., 1917.)
The demonstrable area of pain and temperature loss is typically smaller than the area of light touch loss, and smaller than the published peripheral nerve or dermatome distributions. The deficit to light touch usually corresponds more closely to a nerve distribution than the pinprick loss. In a patient with a focal nerve or root lesions, it may be possible with careful testing to identify a dense zone of severe sensory loss, corresponding to the area of autonomous supply, surrounded by areas of milder sensory loss in the zone of overlap with adjacent nerves (Figure 36.4). Occasionally, there is spread of sensory loss beyond the field of an injured nerve. Patients may have allodynia or hyperpathia in the area of sensory loss. The sensory and other neurologic abnormalities associated with lesions of specific nerves are described in Chapter 46. The sensory examination is important in the diagnosis of peripheral nerve injuries and in the evaluation of progress in nerve regeneration. In disorders of the brachial and lumbosacral plexus, sensory loss follows the same principles as in focal neuropathy but is localized to some plexus component, for example, lateral shoulder in upper brachial plexopathy and medial forearm and hand in lower brachial plexopathy.
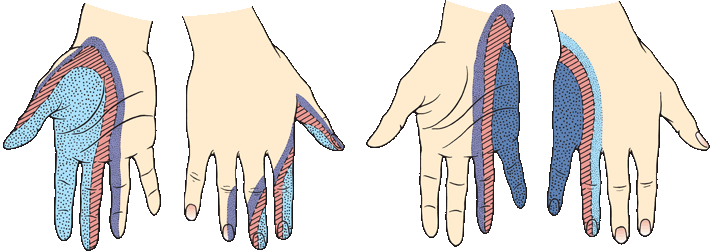
FIGURE 36.4 Transition in sensory changes with lesions of the median and the ulnar nerve. The smallest area is completely anesthetic, the next area has decreased sensation, and the surrounding area has only slight decrease in sensation. (Modified from Tinel J. Nerve Wounds. Rothwell F [trans]. New York, William Wood & Co., 1917.)
In generalized peripheral neuropathies, vibration is often the first modality affected, but in severe cases, all exteroceptive, proprioceptive, and combined modalities are impaired. Some generalized peripheral neuropathies are purely sensory and some purely motor, but most are sensorimotor. Most axonopathies are length dependent, and the distribution of sensory loss usually involves predominantly the distal segments, causing a stocking-glove distribution of blunted sensation. However, margins of the involved area may be poorly demarcated, with no sharp border between the normal and hypesthetic areas. When severe, length-dependent axonopathies cause sensory loss in a strip over the anterior trunk because of involvement of intercostal nerves (shield or cuirass pattern). Even more severe dying back may cause “beanie cap” sensory loss, or global sensory loss, sparing only a strip in the posterior midline. Leprosy sensory loss may be limited to acral, temperature-dependent regions. Axonopathies produce length-dependent reflex loss; ankle jerks first and then more proximal reflexes disappear as the disease progresses.
Demyelinating neuropathies usually cause only slight sensory loss, and the reflexes are lost globally. Rare neuropathies, for example, Tangier’s disease and porphyria, have a predilection for short fibers.
Some generalized neuropathies have a predilection to involve predominantly large or small fibers. Large fiber sensory neuropathies include uremia, Sjogren’s syndrome, vitamin B12 deficiency, certain toxins (pyridoxine, cisplatin, metronidazole), and some cases of diabetes mellitus (pseudotabes). Small fiber neuropathies include amyloidosis, hereditary sensory autonomic neuropathy, and some cases of diabetes mellitus (pseudosyringomyelia). Large fiber neuropathies are typically associated with reflex loss and, when severe, with motor involvement. Small fiber neuropathies typically produce burning pain with no motor loss and preserved reflexes. Peripheral nerve disease may also cause paresthesias, or pain that is either constant or lancinating in character. The nerves themselves may be sensitive and tender to palpation, and there may be pain on brisk stretching of the affected nerves and increased susceptibility to ischemia. There sometimes is hyperalgesia or allodynia in the involved area, even though the sensory threshold is raised.
Disease of the dorsal root ganglia (DRG), or corresponding cranial nerve ganglia, is also associated with sensory changes. The DRG may be affected by autoimmune processes, causing degeneration and inflammation of the neurons. Patients have the subacute onset of pain, paresthesias, and sensory loss, which affects large more than small fibers. Strength is preserved, but reflexes disappear. There is often a disabling sensory ataxia, which may be accompanied by pseudoathetosis. Cerebrospinal fluid protein is frequently increased. Although classically a remote effect of small cell carcinoma of the lung, sensory neuronopathy is associated with a number of other conditions, including pyridoxine intoxication, Sjogren’s syndrome, and lymphoma. In herpes zoster, there is severe, lancinating pain in the distribution of the affected ganglia. The now rare tabes dorsalis causes impairment of deep and superficial pain sensation. Transient, spontaneous “lightning” pains may develop.
Lesions of the nerve root, most often due to compression, are accompanied by diminution or loss of sensation, pain, or paresthesias, but the distribution is segmental and corresponds to the involved dermatome (Figure 36.5). As with focal neuropathy, in compressive radiculopathy, the touch deficit is larger and often corresponds better to the published dermatome than the pinprick deficit. Pain may be either constant or intermittent and is often sharp, stabbing, and lancinating. It is increased by movement, coughing, or straining. There may be either hypalgesia or hyperalgesia. Examination may disclose root compression signs (see Chapter 47). Because of dermatome overlap, sensory changes may be difficult to demonstrate if only one root is involved.
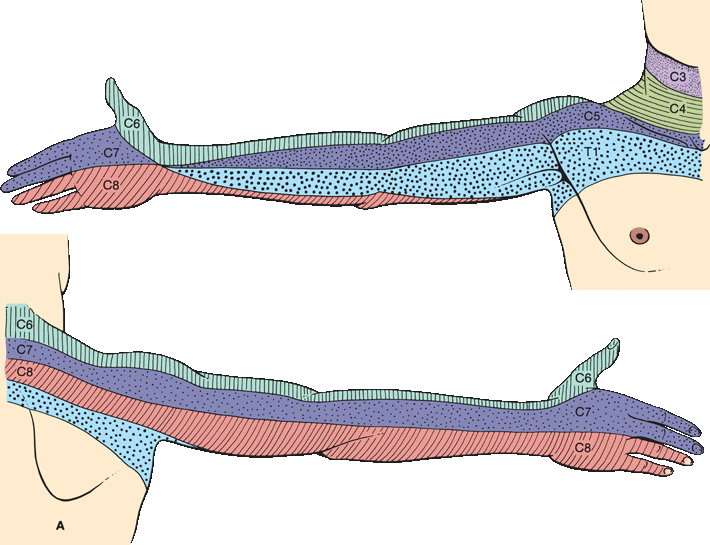
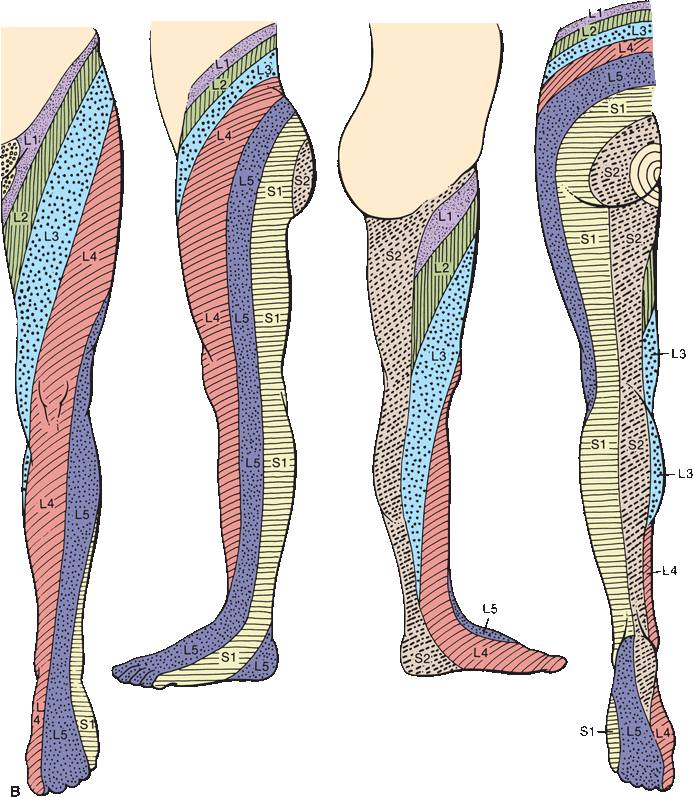
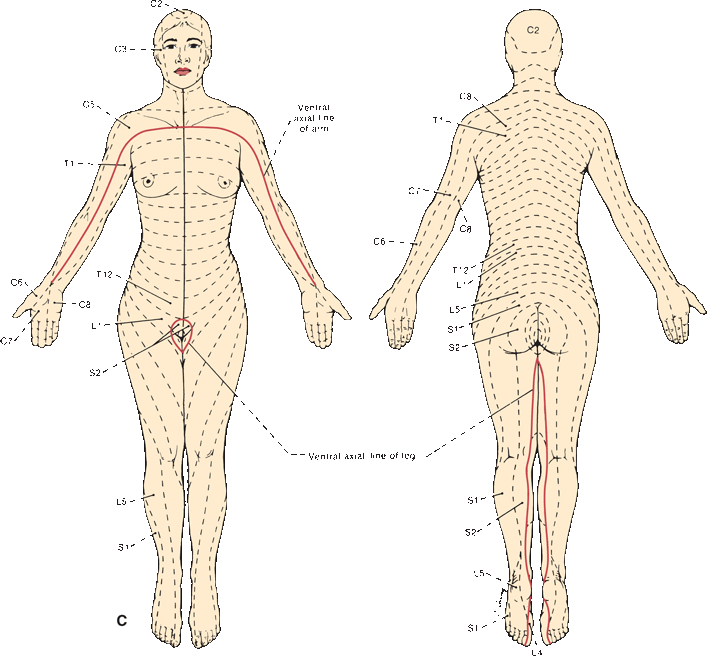
FIGURE 36.5 The segmental innervation. A. The upper extremity. B. The lower extremity. C. The anterior and posterior aspects of the entire body. (Modified from Keegan JJ, Garrett FD. The segmental distribution of the cutaneous nerves in the limbs of man. Anat Rec 1948;102:409–437.)
With lesions of the spinal cord and brainstem, impairment of one or more modalities of sensation, or perversions of sensation in the form of either pain or paresthesias, may develop. Different sensory patterns may occur with myelopathy, for example, transverse syndrome, central cord syndrome, posterior column syndrome, Brown-Séquard syndrome, anterior cord syndrome, or conus medullaris syndrome (see Chapters 24 and 47). With a transverse cord syndrome, the area of sensory involvement may involve all levels below the lesion, but occasionally, the sensory level is well below the level of the lesion; a sensory level on the trunk has been reported in lesions of the lower brainstem. Band-like radicular pain, paresthesias, or sensory loss may occur at the level of the lesion. Sacral sparing may be seen with intramedullary lesions. Spondylotic compression of the cervical spinal cord may cause glove-distribution sensory loss in the hands. Sensory loss is usually dissociated, with impairment of certain modalities and sparing of others. Because of the redundancy of the touch pathways, pain and temperature testing may be more useful than tactile sensation in evaluating CNS disease. Testing for the ability to detect the direction of skin movement above and below the level of the lesion and searching for a vibratory level may be helpful. Suspended dissociated sensory loss occurs in syringomyelia.
Lesions high in the cervical spinal cord and in the medulla may impair kinesthetic sensation in the upper extremities more than in the lower. As a result of the disturbance of proprioceptive sensations and a raised threshold for cutaneous senses, there may be stereoanesthesia, a term occasionally used when the difficulty results from infracerebral lesions, which is difficult to differentiate from astereognosis. Extinction and even autotopagnosia may be present with such lesions. Patients with pontine, medullary, or spinal cord lesions occasionally experience “central” pain. Lhermitte’s sign, sudden electriclike or painful sensations spreading down the body or into the back or extremities on flexion of the neck due to involvement of the posterior columns, may occur with focal lesions of the cervical cord, multiple sclerosis, or other degenerative processes.
The pattern of sensory return with recovering spinal lesions is variable; the impairment may recede downward in a segmental manner; the return may start in the sacral distribution and ascend, or there may be a gradual recovery of function over the entire affected area. Pressure sensation returns first and its recovery is usually the most complete, followed, in turn, by tactile, pain, cold, and heat sensibilities.
Sensory impulses that enter consciousness for interpretation by the parietal cortex must first pass through the thalamus. The thalamus is thought to be the end-station for pain, heat, cold, and heavy contact, where sensory impulses produce a crude, uncritical form of perception. Thalamic lesions usually cause impairment of all sensory modalities on the opposite side of the body. Small lesions limited to the ventral posterior lateral nucleus may cause paresthesias without demonstrable sensory loss. Severe and extensive lesion may cause gross impairment of all forms of sensation. Marked loss of appreciation of heavy contact, posture, passive movement, and deep pressure perception occurs, and the thresholds for light touch, pain, and temperature sensations are raised. Thalamic lesions are often associated with sensory perversions, such as paresthesias and hyperesthesias, or painful hyperpathia or allodynia. Some thalamic lesions may blunt cold but not heat sensation.
In the thalamic pain (Dejerine-Roussy) syndrome, there is blunting, or raising of the threshold, of all forms of sensation on the opposite side of the body, without complete anesthesia. The latency to detection may also be raised. Suprathreshold stimuli excite unpleasant sensations, and any stimulus, even the lightest, may evoke a disagreeable, often burning, pain. Slight hot and cold stimuli, or light cutaneous sensations, cause marked discomfort. The overreaction is termed hyperpathia, hyperalgesia, or allodynia depending on the stimulus. Impairment of sensation accompanied by intractable pain in the hypesthetic regions is called anesthesia dolorosa. In addition to the sensory changes, hemiparesis and hemianopia usually occur and, less frequently, hemiataxia, choreoathetosis, and unmotivated emotional responses. Pain of central origin is most often associated with thalamic lesions but may occasionally result from involvement of other central pain pathways. Central pain due to a parietal opercular lesion has been termed the pseudothalamic syndrome. Occasionally, pleasurable stimulation, such as application of a warm hand to the skin on the affected side, may be markedly accentuated. This overreaction is due to a thalamic lesion or to release of thalamic function from normal cortical control by damage to higher centers. Every stimulus acting on the thalamus produces an excessive effect on the abnormal half of the body, especially as far as the affective element—the pleasant or unpleasant character in its appreciation—is concerned.
In a series of 25 patients with thalamic stroke, 9 had a loss of all modalities of sensation with facio-brachiocrural distribution, 5 suffered dissociated sensory loss with faciobrachiocrural distribution, 11 showed a dissociated involvement of sensation with a partial distribution pattern, 18 had contralateral paresthesias, 6 complained of pain and/or dysesthesias during the stroke, and 4 developed delayed pain and/or dysesthesias
Involvement of the sensory radiations in the posterior limb of the internal capsule causes variable, sometimes extensive, impairment of all types of sensation on the opposite side of the body. Because the sensory fibers are crowded closely together, the sensory loss is more severe than with isolated cortical lesions. The changes are similar to those that follow a thalamic lesion, but pain is rare.
Lesions of the parietal cortex rarely cause complete loss of sensation, but there is a raising of the threshold for both exteroceptive and proprioceptive sensations of the opposite side of the body. Sensation is often disturbed more in the upper than in the lower extremity. The distal parts of the extremities are affected more than the proximal portions, with a gradual transition to more normal perception approaching the shoulder and hip. Involvement of the hand and face is common because of their extensive cortical representation. Small lesions may produce restricted deficits simulating peripheral nerve or root pathology.
Parietal lesions primarily cause disturbances in discriminatory sensation. Detailed and critical examination of sensory functions may be necessary to detect parietal lobe lesions. The threshold for pain stimuli is raised very little in parietal lesions, although a prick may feel less sharp than on the normal side; with deeper lesions, the threshold is more definitely raised. Qualitative appreciation of heat and cold are present, but there is loss of discrimination for slight variations in temperature, especially in the intermediate ranges. Light touch perception is little disturbed, but tactile discrimination and localization may be profoundly affected. There often is severe impairment of position sense resulting in sensory ataxia and pseudoathetosis, but vibratory sensation is only rarely affected (another instance where vibration and position sense loss are dissociated). Astereognosis is common, but both small and large objects may have to be used to detect the deficit; sometimes a delay in answering when objects are placed in the affected hand, with no delay with the other hand, may be a clue to minimal involvement. Bilateral simultaneous testing for stereognostic sense, placing identical objects in both hands, may be useful. Sensory inattention, or extinction, is often an early and important diagnostic finding in parietal lobe lesions (see Chapters 10 and 35). Other possible findings include abarognosis, agraphesthesia, impairment of two-point discrimination, autotopagnosia, anosognosia, or Gerstmann’s syndrome. The ability to distinguish two cutaneous stimuli to the same side of the body but separated by a brief time interval is also impaired with parietal lobe lesions.
In a series of 20 patients with stroke limited to the parietal lobe, three main sensory syndromes were found: (a) a pseudothalamic sensory syndrome consisting of faciobrachiocrural impairment of elementary sensation (touch, pain, temperature, vibration) due to a lesion involving the inferior-anterior parietal cortex, parietal operculum, posterior insula, and underlying white matter; (b) a cortical sensory syndrome consisting of isolated loss of discriminative sensation (stereognosis, graphesthesia, position sense) involving one or two parts of the body due to a lesion of the superior-posterior parietal cortex; and (c) an atypical syndrome with sensory loss involving all modalities of sensation in a partial distribution, likely a variant of the other two sensory syndromes.
Spontaneous discharges from the parietal cortex frequently cause contralateral paresthesias that may constitute a focal sensory seizure or the sensory aura preceding a jacksonian motor convulsion. Only rarely do spontaneous discharges from the parietal cortex cause pain.
NONORGANIC SENSORY LOSS
Nonorganic sensory abnormalities are usually areas of decreased sensibility. Areas of hypesthesia, hypalgesia, anesthesia, and analgesia are commonly encountered that may be complete or partial, affect all modalities, or be dissociated. Even normal individuals, or those with organic sensory loss, may be suggestible and have spurious sensory findings.
One of the obvious clues that sensory loss is nonorganic is failure to follow any sort of anatomical distribution. The demarcation between normal and abnormal often occurs at some strategic anatomical point that has no neurologic significance, such as a joint or skin crease, causing a finding such as numbness circumferentially below the elbow, wrist, shoulder, ankle, or knee. Nonorganic facial sensory loss often stops at the hairline and angle of the jaw, a nonanatomic distribution. A real spinal sensory level on the trunk slants downward from back to front, a functional level may be perfectly horizontal. The term stocking-glove sensory loss is used to describe both hysteria and peripheral neuropathy. The key to understanding this confusing usage is the type of stocking. When sensory loss due to length-dependent peripheral neuropathy extends to about the level of the knees, it appears in the hands, causing loss in a glove-knee sock distribution; with hysteria, the impairment may be distal to the wrists and ankles: a glove-ankle sock distribution. The border between normal and abnormal is usually abrupt and well demarcated, more discrete than in organic sensory loss, and may vary from examination to examination, or even from minute to minute. Sensation may be different on the ventral and dorsal surfaces. Responses are typically inconsistent. In spite of complete loss of cutaneous sensibility, the patient may have intact stereognosis and graphesthesia, or in spite of complete loss of position sense may be able to perform skilled movements and fine acts without difficulty, and have no Romberg sign. On finger-to-nose testing, the examiner may touch one finger of the “anesthetic” hand and ask the patient to touch her nose with it; a patient with organic exteroceptive sensory loss will not know which finger was touched, while those with organic proprioceptive loss cannot find their nose. The hand wandering widely before eventually finding the nose suggests histrionic tendencies. In the search test, the patient holds the involved hand in the air and searches for it with the unaffected hand. In nonorganic loss, there may be no difficulty, but with bona fide proprioceptive loss, performance is poor with either hand.
Clinical subterfuge is often used to establish that sensory loss is nonorganic. The author has seen all of these “tricks” fail (i.e., indicate the sensory loss is not real when it is) at one time or another, save one: the SHOT syndrome. In the SHOT syndrome, the patient claims to have no Sight in the eye, no Hearing in the ear, no Olfaction in the nose, and no Touch sensation on the body, all on the same side. This pattern is of course utterly impossible on an anatomic basis and its presence reliably indicates that hemibody numbness is nonorganic. Another sometimes helpful technique to bring out nonorganic sensory loss is the “yes if you feel it/no if you don’t” maneuver. After demonstrating the patient is unable to feel a given stimulus in a given distribution, instruct her to close her eyes and say “yes” every time she feels a stimulus and “no” when she does not; the gullible will respond with “no” every time the alleged anesthetic region is stimulated. A medical student once asked, “How did she know to say ‘no’ if she didn’t feel it?”
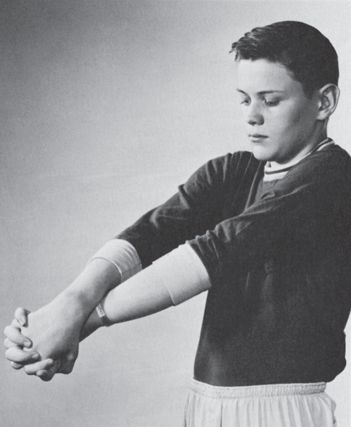
It is often possible to confuse the patient and confirm the absence of organic changes by checking sensation while the hands are in some bewildering position where it is difficult to tell which side is which, such as crossed behind the back or intricately entwined. A commonly used technique is to have the patient cross the hyperpronated forearms and hold the hands with little fingers up, palms together, and fingers interlocked. The hands are then rotated downward and inward, then upward, so that the little fingers are facing the chest (crossed hands test, Figure 36.6). Anyone who has ever done this knows how difficult it is to tell which finger belongs to which hand. The patient responds as digits are stimulated randomly. It matters little whether eyes are open or closed, and in fact, the test may work better with eyes open. The patient with nonorganic hemianalgesia may make errors, while the one with organic loss will not. The nonorganic patient may respond slowly, delay answering, or betray signs of the effort required. It is of course imperative that the examiner accurately keep track of which side is which. With practice, performance improves rapidly, so the test is most conclusive the first time it is done.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







