Sleep
Margaret N. Shouse
Carl W. Bazil
Beth A. Malow
Introduction
Sleep states influence the expression or suppression of seizures. The two main sleep states, non–rapid-eye-movement (NREM) sleep and rapid-eye-movement (REM) sleep, have different physiologic components and contrasting effects on generalized ictal and interictal discharges (IIDs). Epileptiform discharges are likely to propagate during NREM sleep and its transitional EEG “drowsy” periods. In contrast, REM sleep is resistant to the propagation of epileptic potentials even though focal IIDs persist at this time.
This chapter describes the distribution of ictal and IID events during NREM versus REM sleep, differentiates NREM from REM sleep substrates, and suggests how the neural generators for seizure-prone or seizure-resistant sleep states modulate the timing of seizures. We also discuss the effects of seizures, antiepileptic drugs (AEDs), and vagus nerve stimulation on sleep. Finally, we consider the impact of sleep disorders and sleep deprivation on seizures.
Seizures and Interictal Discharges in Non–Rapid-Eye-Movement Versus Rapid-Eye-Movement Sleep
Clinical Findings
During NREM sleep, focal and generalized epileptic discharges are common, even if clinical seizures do not occur.52,78,95 During REM sleep, generalized epileptiform discharges are infrequent. Focal interictal discharges are less numerous but persist during REM sleep and are highly localized (e.g., Sammaritano et al.113). Seizures are common during NREM sleep but rarely occur during REM sleep.53,93,95 The effects of NREM sleep versus REM sleep on interictal and ictal epileptiform discharge depend somewhat on the epileptic syndrome (Table 1).
Primary Generalized Epilepsies of Genetic or Idiopathic Origin
In the primary generalized epilepsies (PGEs), interictal and ictal discharges begin simultaneously in all or most of the cerebral cortex bilaterally.25,48,95 Most PGEs are thought to be genetic with age-related penetrance (e.g., Delgado Escueta et al.32 and Niedermeyer95). Examples are childhood and juvenile absence epilepsy and juvenile myoclonic epilepsy (JME), which can occur with or without primary generalized tonic–clonic convulsions2,25,95 (see Chapters 239, 240, and 244).
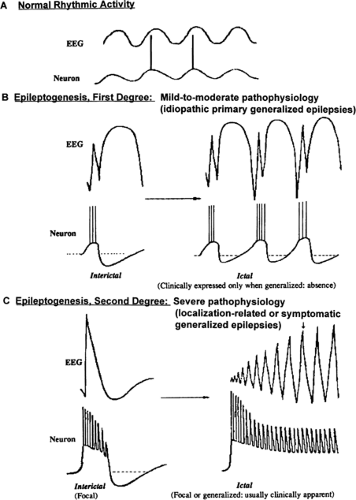
These primary generalized epilepsies presumably result from a diffuse abnormality of cortical excitability that is less severe than that seen with a focal epileptogenic lesion.48,49 This concept is illustrated in FIGURE 1, which contrasts the surface electrographic and intracellular discharge patterns in cortex associated with normal rhythmic activity (Fig. 1A), PGE (Fig. 1B), and localization-related epilepsies (Fig. 1C).
In PGE, the membrane potentials of individual cells are within normal range, and intracortical inhibitory mechanisms are generally intact. The underlying neuronal hyperexcitability is expressed only in populations of cortical neurons entrained to a synchronous burst–pause firing pattern by the glutaminergic and aspartate-mediated thalamocortical volleys that normally evoke spindles and recruiting responses. Sequences of cortical excitatory postsynaptic potentials (EPSPs) alternate with inhibitory postsynaptic potentials (IPSPs) to generate spindles and recruiting responses. The sequential pattern persists in PGE but is altered by the increased amplitude of temporally and spatially summed EPSPs and IPSPs. The enhanced population excitatory response generates the action potentials underlying the spike or multispike component and is followed by a longer-lasting period of neuronal silence that gives rise to the slow-wave component of the complex (Fig. 1B, left). Preservation of γ-aminobutyric acid (GABA)-mediated intracortical inhibitory mechanisms is considered critical to the generation of the slow component and terminates the spike train, which, if unopposed, could lead to a convulsion (Fig. 1B, right).
Interictal and ictal discharges often arise during transitional “drowsy” arousal periods, which occur throughout the sleep–wake cycle.52,57,95 For example, awakening from sleep presents a high risk for the occurrence of IIDs and/or ictal seizure events in idiopathic generalized epilepsies such as awakening “grand mal” seizures and juvenile myoclonic epilepsy. Such periodicities imply mediation of seizure events by both circadian and ultradian cycles. Chronobiology issues are primarily covered in Chapter 187.
This chapter focuses mainly on stable sleep, defined by the physiologic components that persist after initial onset of a specific NREM or REM sleep stage (e.g., Carskadon and Dement21) vis-à-vis IID and ictal events. Below, we describe sleep-related interictal and ictal discharges in this group of seizure disorders for (a) light NREM sleep (stages 1 and 2), (b) deep NREM sleep (stages 3 and 4), and (c) REM sleep.
Light Non–Rapid-Eye-Movement Sleep: Stages 1 and 2.
Interictal discharges, such as typical spike-and-wave (3/sec) and multispike-and-wave or polyspike-and-wave complexes, are more often detected in light NREM sleep (e.g., Niedermeyer95) than in deep NREM sleep or in REM sleep (e.g., Carskadon and Dement21). The longest trains of typical spike-and-wave complexes (>5 seconds) occur in stage 1 NREM sleep. Discharges of similar duration in an awake patient would typically be accompanied by a clinically evident seizure.
The next-most-prominent time for spike-and-wave and multispike- or polyspike-and-wave complexes is stage 2 NREM sleep. During this stage, IID complexes are frequent but of short duration. They usually coincide with sleep transients, which have an EEG configuration similar to epileptiform potentials95 such as sleep spindles, K-complexes, vertex waves, and bursts
of slow waves. Some evidence suggests that these events, particularly K-complexes, reflect aborted arousals during sleep.95 For example, exogenous stimulation during NREM sleep, such as a loud noise, can evoke any of these transient waveforms.
of slow waves. Some evidence suggests that these events, particularly K-complexes, reflect aborted arousals during sleep.95 For example, exogenous stimulation during NREM sleep, such as a loud noise, can evoke any of these transient waveforms.
Table 1 Generalized epileptiform discharges by sleep states in three epilepsy syndromes with convulsions at different times in the sleep–wake cycle | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||
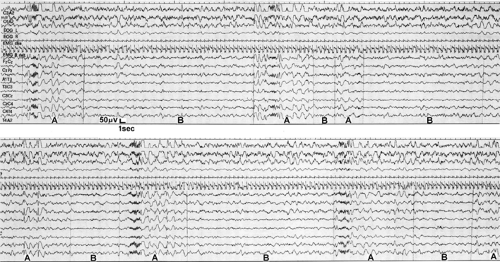 FIGURE 2. Polyspike-and-wave complexes associated with sleep spindles in juvenile myoclonic epilepsy (JME). Spindles and other sleep electroencephalogram (EEG) transients are thought to represent aborted arousals during sleep, and both aborted and actual arousals can provoke seizure events. This conclusion is supported by the higher incidence interictal discharges during the “arousal” phase (A) versus the reduced “arousal “ phase (B) of Terzano’s cyclic alternating patterns142 (see Chapter 187 for more detail) and also by reports (e.g., Touchon143) that induced or spontaneous behavioral arousal from sleep most often provokes clinical seizure manifestations in JME. (Reprinted from Gigli GL, Calia E, Marciani MG, et al. Sleep microstructure and EEG epileptiform activity in patients with juvenile myoclonic epilepsy. Epilepsia. 1992;333:799–804; with permission.) |
Figure 2 shows polyspike-and-wave complexes, which occur in relation to sleep spindles during the “arousal phase” of Terzano’s cyclic alternating pattern (CAP) in a patient with juvenile myoclonic epilepsy (JME). CAP refers to sequences of transient electroclinical events that are distinct from homogeneous background EEG activity in NREM sleep and recur at up to 1-minute intervals.142 See Chapter 187 for an explanation of ultradian rhythms, including CAPs (e.g., Parrino et al.100 and Terzano et al.142). For the purpose of this chapter, note that induced or spontaneous behavioral arousals from sleep most often provoke clinical seizure manifestations in JME (e.g., Touchon143), regardless of CAPs.
Deep Non–Rapid-Eye-Movement Sleep: Stages 3 and 4.
Deep NREM sleep stages are usually considered least likely to activate typical spike-and-wave and multispike-and-wave or polyspike-and-wave complexes. However, some evidence suggests that deep NREM sleep is equally if not more conducive than light NREM sleep to these IIDs.110,117 The IID trains are briefer and less organized in deep NREM than in light NREM sleep. This probably results from the prolonged hyperpolarization of thalamocortical cells1,135,137 that generate the delta-wave oscillation of deep NREM and disrupt the rhythmic burst–pause discharge pattern as well as the morphology of spike-and-wave and multispike-and-wave complexes seen in light NREM sleep.
Rapid-Eye-Movement Sleep.
Localization-Related Epilepsies
This group of seizure disorders is thought to result from one or more focal regions of cerebral dysfunction (e.g., Commission Report25). Localization-related epilepsies are thought to reflect a more severe pathophysiology than that seen in PGE because individual neurons in the focus exhibit a sudden depolarization shift representing summated or giant EPSPs.48 Depolarization shifts are characterized by high-amplitude, long-lasting depolarizations with superimposed high-frequency action potentials corresponding to spikes and sharp waves on the surface. Recurrent GABAergic inhibitory mechanisms are present but impaired, as evidenced by the absence of rebound inhibition during interictal discharge (Fig. 1C, left).48,49 The intense hyperexcitability of focal epileptic neurons could increase the likelihood of seizure discharge propagation in response to synchronous bursts of excitatory synaptic inputs during NREM sleep (Fig. 1C, right).
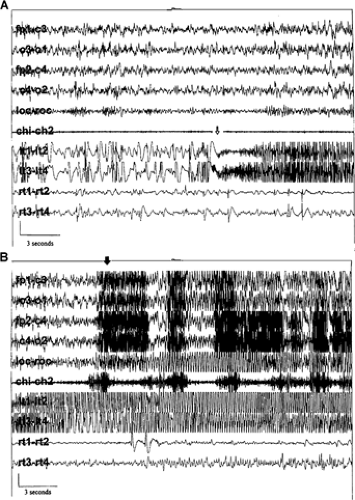
Figure 3 shows that a spindle-like train of high-frequency epileptiform discharge in depth electrodes precedes seizure onset (Fig. 3A) and propagation during NREM in a patient with temporal lobe epilepsy (TLE).73 It was concluded that, unlike PGE, EEG and behavioral arousal events most often follow seizure onset,73 although others have concluded
that localization-related seizures are often precipitated by behavioral or EEG arousals during NREM sleep (e.g., Silvestri et al.134).
that localization-related seizures are often precipitated by behavioral or EEG arousals during NREM sleep (e.g., Silvestri et al.134).
There is significant variation in the severity of specific localization-related epileptic syndromes, based upon differences in medical refractoriness, spontaneous remission, and organicity.91,121,123 Etiologies include but are not limited to trauma (see Chapter 253), lesions (see Chapter 251), and/or genetics (e.g., autosomal-dominant nocturnal frontal lobe epilepsy [ADNFLE] and some temporal lobe epilepsies; see Chapters 248, 249 and 255). Localization-related epilepsies with frontal or temporal lobe foci tend to be associated with more frequent clinically evident seizures, are more likely to be medically refractory119 (although there are notable exceptions; e.g., nocturnal paroxysmal dystonia and ADNFLE), and are less likely to spontaneously remit than are the so-called “benign localization-related epilepsies.” Regardless of these differences, localization-related epilepsies are most likely to display interictal and ictal discharges during NREM sleep.7,8,9,40,53,57,58,78,91,95,121
Table 2 Interictal discharges (IIDs) and visually scored sleep stage in 21 patients with medically refractory temporal lobe epilepsy | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Frontal and Temporal Lobe Epilepsies.
Frontal and temporal lobe epilepsies comprise the largest group of localization-related seizure disorders25,90 (see Chapter 25) and the largest group of “pure sleep epilepsies”:57,58 Up to 59% of patients have been reported to exhibit convulsions mostly or only during sleep.57,91 Recent evidence indicates that secondary generalization during sleep is less prominent in frontal than temporal lobe epilepsies, although 55% of secondary generalized complex partial seizures occur in sleep regardless of onset site or the timing of other seizure types.53 In addition, IIDs, such as anterior spikes or sharp waves, have higher amplitudes, longer durations, and rounder peaks in NREM than in other sleep or waking states (e.g., Frost et al.40).
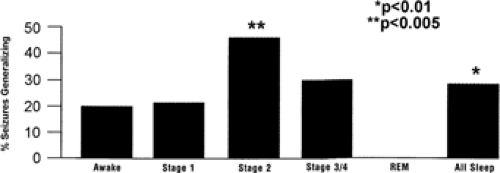
Although both clinical seizures and IIDs are more common in NREM sleep than in REM sleep, evidence suggests that deep NREM sleep is the most vulnerable period for IID,78,113 whereas lighter NREM sleep is the most vulnerable period for clinical seizures.53,93 This dissociation between clinical seizures and IIDs is illustrated by comparing Table 278 to FIGURE 4.53 Table 2 shows that the incidence of IIDs per minute peaks during NREM stages 3 and 4 sleep,78 whereas FIGURE 4 shows a peak in the incidence of secondary generalized seizures in stage 2 sleep.53
Several authors have emphasized a connection between REM sleep and partial or complex partial seizures originating from temporal lobe foci. However, most of these seizures occurred during NREM transitional states to or from REM rather than in stable REM sleep (e.g., Cadhillac19 and Shouse et al.123). Because REM sleep transitions reflect an ultradian rhythm, this topic is described in more detail in Chapter 187. Herman et al.53 saw few focal and no secondary generalized seizures during REM in a large series with 613 partial seizures in 133 patients regardless of seizure onset site, and Minecan et al.93 noted that only 5% of seizures occurred during REM sleep in a separate series with 117 seizures in 55 patients. Neocortical seizures can be an exception (e.g., Mendez and Radtke91). Other evidence of the protective effects of REM sleep include the correlation between elevated REM percentages and reduced seizure incidence in human TLE104 as well as the relative difficulty in evoking seizures20 and the absence of
spontaneous seizures during REM sleep in amygdala-kindled cats.124,125
spontaneous seizures during REM sleep in amygdala-kindled cats.124,125
Benign Localization-Related Seizure Disorders.
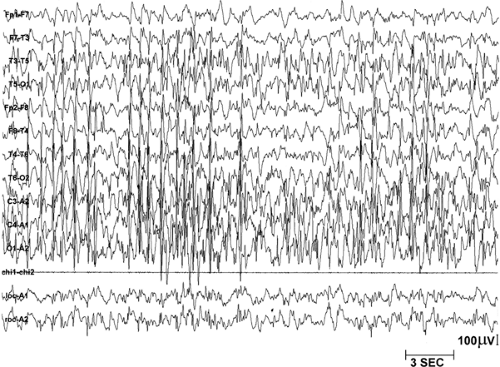
Examples of “benign” localization-related seizure disorders are benign epilepsy with centrotemporal spikes (BECT, also called benign rolandic partial epilepsy; see Chapter 236), benign occipital localization epilepsies (see Chapters 237 and 238), electrical status epilepticus during sleep (ESES; see Chapter 242), and Landau-Kleffner syndrome (acquired epileptic aphasia; see Chapter 242). Like seizure disorders of deep temporal or frontal lobe origin, these epilepsies are associated with diffuse IIDs during NREM sleep and maximal localization during REM sleep. Unlike many seizure disorders of temporal or frontal lobe origin, these childhood seizure disorders are considered benign because the seizures are responsive to AEDs and are likely to remit spontaneously (e.g., Mendez and Radtke91 and Shouse121). Patients may be referred for a variety reasons other than seizures (e.g., slow progress in school in ESES, sudden deterioration of language skills related to auditory aphasia in Landau-Kleffner syndrome), and the seizure disorder may only be noted in a polysomnogram. FIGURE 5 shows an example of ESES, in which spike and wave discharges occupy at least 85% of NREM sleep. These types of pervasive seizure discharges in sleep are hypothesized to generate the nonepileptic symptoms of ESES and Landau-Kleffner syndrome as well, even though clinical seizures are rare and may not occur at all. In both ESES and Landau-Kleffner syndrome, cognitive, social, and linguistic anomalies tend to mitigate with seizure remission, but residual “nonepileptic” effects often persist (e.g., Mendez and Radtke91 and Shouse et al.121).
Interictal Discharge Versus Ictal Seizure Discharge Propagation During Non–Rapid-Eye-Movement Sleep.
Differences in ictal seizure discharge propagation patterns cannot entirely be attributed to whether or not the localization-related seizure disorder is “benign.” An unanswered question is why IIDs, which
spread during NREM sleep, do not end in secondary generalized seizures. One possibility is the apparent location of the seizure focus. For example, many of the “benign” localization-related epilepsies appear to have neocortical foci. Ictal seizure discharge propagation seems to occur less often from neocortical foci than from subcortical sites during NREM sleep, as described previously. The presence of “inhibitory surrounds” has long been invoked to explain the confinement of cortical interictal and ictal discharges. This concept has recently been elaborated to include mechanistic interactions between glial and neuronal cell populations in neocortex and in deep cortical sites such as the hippocampus (e.g., Amzica and Steriade1). On the other hand, Herman et al.53 found that seizures of mesial and neocortical temporal lobe origin exhibit secondary generalization more often than do those of frontal neocortical origin. See FIGURE 4 and the discussion of different findings between Herman et al.53 and Crespel et al.28 in Chapter 187. Another complication is the difficulty in verifying the actual site of the seizure focus and/or the propagation patterns even when subcortical leads are available. A third complication is that rapid seizure discharge propagation can occur in symptomatic localization-related syndromes, particularly those with neocortical tumors, and may initially present as primary generalized seizures (e.g., Malow et al.79).
spread during NREM sleep, do not end in secondary generalized seizures. One possibility is the apparent location of the seizure focus. For example, many of the “benign” localization-related epilepsies appear to have neocortical foci. Ictal seizure discharge propagation seems to occur less often from neocortical foci than from subcortical sites during NREM sleep, as described previously. The presence of “inhibitory surrounds” has long been invoked to explain the confinement of cortical interictal and ictal discharges. This concept has recently been elaborated to include mechanistic interactions between glial and neuronal cell populations in neocortex and in deep cortical sites such as the hippocampus (e.g., Amzica and Steriade1). On the other hand, Herman et al.53 found that seizures of mesial and neocortical temporal lobe origin exhibit secondary generalization more often than do those of frontal neocortical origin. See FIGURE 4 and the discussion of different findings between Herman et al.53 and Crespel et al.28 in Chapter 187. Another complication is the difficulty in verifying the actual site of the seizure focus and/or the propagation patterns even when subcortical leads are available. A third complication is that rapid seizure discharge propagation can occur in symptomatic localization-related syndromes, particularly those with neocortical tumors, and may initially present as primary generalized seizures (e.g., Malow et al.79).
Longitudinal Course of Sleep Epilepsies.
In temporal or frontal lobe epilepsies, seizures may occur initially only during sleep, although a random seizure pattern can develop later in the clinical course (e.g., D’Alessandro et al.29). For example, Janz reported that 38% of 100 patients with a 2-year or shorter history of epilepsy manifested complex partial seizures only during sleep, but a large percentage of these patients soon developed diurnal seizures as well.58 Other studies45,99 used patients with primary generalized, secondary generalized, and partial seizures that occurred only in sleep epilepsy at initial diagnosis. Over a 2-year follow-up, they found that patients with generalized seizures exhibited few seizures and tended to remit or to retain a sleep seizure pattern, whereas those with focal seizures tended to develop waking seizures as well. Effective medical management may also cause seizures to disperse across the sleep–wake cycle.63
The degree to which seizure patterns are state dependent may predict response to medication. Frequent seizures that are entrained to a specific sleep or arousal state usually respond better to medical treatment than epilepsies in which seizures are randomly distributed across the sleep–wake cycle.55,84,99,152 Particular epilepsy syndromes are associated with different patterns of seizure occurrence. For example, benign or idiopathic localization-related epilepsies are more likely to retain nocturnal seizure patterns than are localization-related epilepsies with a documented lesion.29,42,58
The reason seizure patterns become random is uncertain. Frequent seizures may exacerbate cell dysfunction as well as response to synaptic input at the seizure focus and can ultimately influence cell discharge patterns in extrafocal brain areas (e.g., McNamara90 and Shouse et al.123). Cells in certain limbic system areas, such as the hippocampus, are particularly sensitive to cerebral insult (e.g., McNamara90). Epilepsies in which there is widespread or progressive cerebral dysfunction usually have random distribution of interictal discharges and clinical seizures throughout the sleep–wake cycle.16,42,44,50,57,58,84,98
Regardless of the timing of clinically evident partial seizures, the rate, amplitude, and spread of IIDs peak during NREM sleep. Diffusion of spikes decreases during waking and further declines during REM sleep (e.g., Frost et al.40). The rate and amplitude of spikes may also be lower in REM sleep than in waking.65,95,113 Because most partial seizure disorders are associated with focal cerebral dysfunction, it is not surprising that autonomous discharges persist during alert waking and REM sleep. Some authors suggest that optimal “focalization” occurs during REM, as seen in FIGURE 6.113 Localizing epileptogenic foci during REM may assist in targeting the epileptogenic region for surgical resection.71,113,114
Symptomatic Generalized Epilepsies
This third group of seizure disorders is associated with variable degrees of diffuse cerebral dysfunction that can disrupt sleep, dissociate its components, and disperse IID and ictal discharges throughout the sleep–wake cycle (see footnote b of Table 1). Examples are West42,54,98,159 (see Chapter 229) and Lennox-Gastaut44,84,98 (see Chapter 241) syndromes and progressive myoclonic epilepsies such as Lafora-Unverricht-Lundborg syndrome16,50 (see Chapter 252).
When NREM and REM states can be differentiated from waking, there is sleep-state modulation of IID and ictal seizure manifestations (e.g., Fukuyama et al.42). For example, hypsarrhythmia, the characteristic EEG manifestation of infantile
spasms (West syndrome; Fig. 7), and the atypical slow spike-and-wave discharges of Lennox-Gastaut syndrome show a peak in NREM and subside during intact REM sleep. The prognosis for response to medical treatment is improved if IID or clinical seizures exhibit a sleep–waking state dependency, even if it is confined to a suppression of overt seizure activity in REM sleep.42,54,55,84
spasms (West syndrome; Fig. 7), and the atypical slow spike-and-wave discharges of Lennox-Gastaut syndrome show a peak in NREM and subside during intact REM sleep. The prognosis for response to medical treatment is improved if IID or clinical seizures exhibit a sleep–waking state dependency, even if it is confined to a suppression of overt seizure activity in REM sleep.42,54,55,84
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree