Chapter 88 Sleep and Stroke
Stroke
Stroke is defined as a focal neurologic deficit of acute onset and vascular origin. Stroke has an incidence of 2 or 3 per 1000 per year and is the most common neurologic disease to warrant hospitalization. Transient ischemic attacks (TIAs), in which neurologic deficits resolve within 1 hour, account for about 20% of acute cerebrovascular deficits and intracerebral hemorrhage for about 15%. The remaining 65% are ischemic strokes. Risk factors for stroke include atrial fibrillation, age older than 65 years, arterial hypertension, heart diseases, asymptomatic carotid stenosis, history of TIA, alcohol abuse, smoking, diabetes mellitus, and hypercholesterolemia. However, these factors explain only about 50% of the cardiovascular disease risk. Inflammatory changes, infection, homocysteine, and SDB represent new risk factors for stroke.1 According to the criteria of the TOAST study (Trial Of Acute Stroke Treatment), the etiology of stroke can be grouped into five categories: macroangiopathy (large-vessel disease), microangiopathy (small-vessel disease), cardioembolism, other etiologies (e.g., dissection, hypercoagulable states, vasculitis), and unknown origin.2
Primary prevention of stroke includes treatment of risk factors (including hypertension and hypercholesterolemia), regular physical exercise, reduction of body mass index to less than 25, anticoagulation for atrial fibrillation, and endarterectomy in patients with 70% or more carotid stenosis.3,5 Treatment of acute stroke includes admitting patients to a stroke unit, recognizing medical complications early, and prescribing agents that inhibit platelet aggregation.3 Fibrinolytic agents are effective in the first 4.5 hours after ischemic stroke.4 Surgery may be considered in patients with accessible (e.g., cerebellar) hemorrhages. After stroke, prevention of further events includes antiaggregants, antihypertension medications, statins, treatment of risk factors, and—in selected patients—anticoagulation and endarterectomy.3
History
Changes in sleep and breathing were reported in stroke patients from the beginning of the 19th century. In 1818 John Cheyne described periodic breathing in a patient with cardiac disease and “apoplexy.”6 Hughlings Jackson recognized that Cheyne-Stokes respiration often accompanies bilateral hemispheric stroke. Symptoms of obstructive sleep apnea (OSA) were recognized in a patient with intracerebral hemorrhage by Broadbent in 1877.7 Charles Beevor described the loss of voluntary respiratory control in a patient with pseudobulbar palsy (cited in Oppenheim’s book8). Although central apnea in the course of progressive paralysis was described already by Weir Mitchell in 1890,9 failure of automatic respiration during sleep (later called “Ondine’s curse”) after brainstem stroke was first reported by Ratto in 1955.9
Hypersomnia following stroke was mentioned by MacNish in 1830,10 but it was only at the beginning of the 20th century that thalamic and mesencephalic stroke, in particular, were implicated.11,12 In 1883 Charcot reported that a patient lost dream recall after a presumed parieto-occipital stroke.13 Lhermitte joined the term peduncular hallucinosis in 1922 to describe vivid, colorful, and dreamlike hallucinations following midbrain stroke.14 A first report on sleep electroencephalographic (EEG) changes following stroke appeared in 1948.15
Sleep-Disordered Breathing and Stroke
Epidemiology
Sleep-Disordered Breathing as Risk Factor for Stroke
Habitual snoring—that is, snoring occurring always or almost always—affects 4% to 24% of the adult population, with a maximum prevalence around the age of 50 to 60 years, and is strongly associated with OSA.16 Five case-control and three cohort studies have shown that habitual snoring represents an independent risk factor for stroke, with a pooled risk of about 1.5.17
Longitudinal and population studies have suggested a direct link between polysomnographically proven SDB and stroke. In a study of 1022 patients admitted to a single sleep center and followed up over up to 6 years, Yaggi and colleagues found that OSA is associated with an increased risk for stroke and death, even after adjusting for cardiovascular risk factors, such as arterial hypertension, diabetes mellitus, atrial fibrillation, hyperlipidemia. and smoking status, with an hazard ratio (HR) of 2.0 (95% confidence interval [CI], 1.12 to 3.48). The risk was even higher (HR, 3.3; 95% CI, 1.74 to 6.26) in patients with severe OSA (apnea–hypopnea index [AHI] > 36/hr).18
In a single-center study of 1387 male patients with OSA, 377 simple snorers, and 264 controls, matched for age and body mass index (BMI), Marin and coworkers found, after a mean follow-up period of 10 years, a significantly higher incidence of fatal and nonfatal cardiovascular events (e.g., stroke, myocardial infarction, coronary artery bypass graft surgery, percutaneous transluminal coronary angiography) in patients with severe OSA (AHI > 30/hr) as compared to patients with mild to moderate OSA, patients with OSA of any severity treated with continuous positive airway pressure (CPAP), simple snorers, and controls.19 Multivariate statistics, adjusting for potential confounders (hypertension, diabetes, cardiovascular disease, lipid disorders, smoking status) showed that untreated severe OSA increased the risk of fatal and nonfatal cardiovascular events compared with healthy participants.
In a cross-sectional analysis of 1475 subjects, Arzt and colleagues showed that those with an AHI of at least 20/hr have an odds ratio (OR) of 4.3 (95% CI, 1.32 to 15.24) compared to those with an AHI less than 5/hr, even after adjustment for known risk factors.20
In an elderly population of 394 noninstitutionalized stroke-free subjects (>70 years), Munoz’s group showed after a follow-up of 6 years that persons with severe SDB (AHI > 30/hr) have an increased risk for stroke with a hazard ratio of 2.5 (95% CI, 1.04 to 6.01) even when data are adjusted for sex.21
A link between SDB and silent stroke or white matter disease on magnetic resonance imaging (MRI) has also been examined.22–25 In a study of 50 OSA patients, Minoguchi and colleagues found an increased risk for silent brain infarction (as detected by MRI) for those with an AHI of at least 30 when compared with weight-matched patients with mild or no OSA.25
Sleep-Disordered Breathing in Stroke Patients
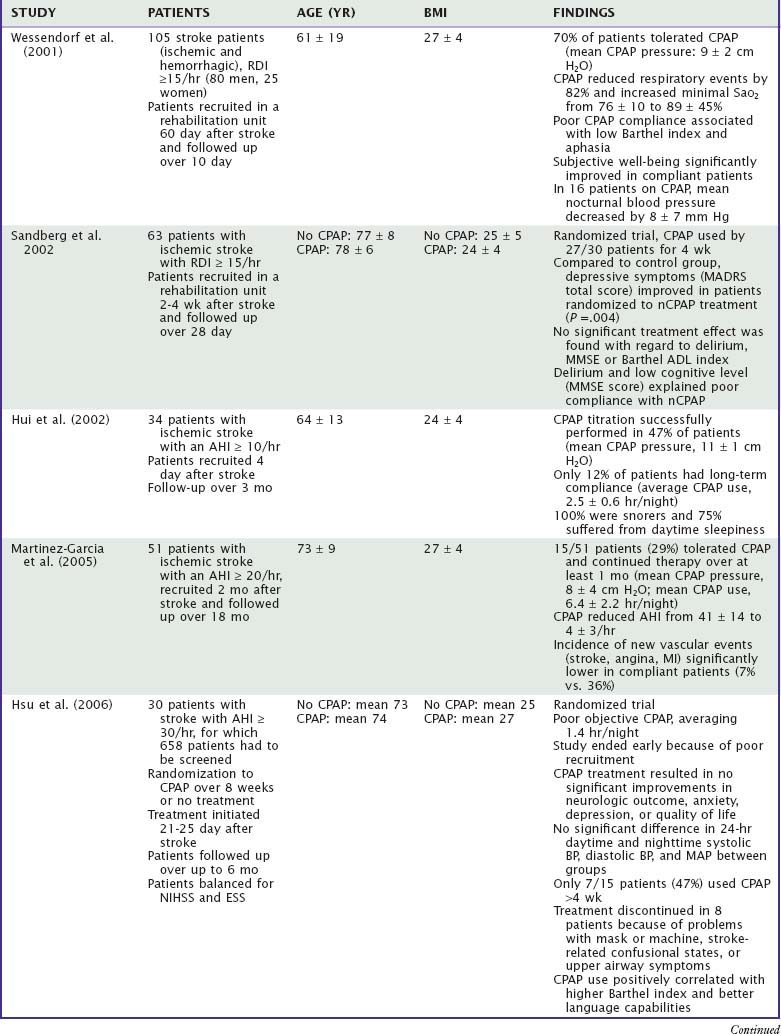
More than 20 studies (Table 88-1) on almost 2000 patients have shown, despite different diagnostic approaches (polysomnography or respirography), that at least 50% of stroke patients exhibit SDB, as defined by an AHI of 10/hr or more.26–49 Few studies suggested a higher incidence of SDB depending upon type (hemorrhagic versus ischemic), presumed etiology (macroangiopathy versus other origins), and severity or clinical characteristics (facial or bulbar involvement, recurrent versus first-ever event) of stroke. In seven studies, the prevalence of SDB was found to be similar also in patients with TIAs and ischemic stroke (see Table 88-1).26,27,31,38,44,51a,51b
In a 2010 metaanalysis of 29 articles (2343 ischemic or hemorrhagic stroke and TIA patients), the frequency of SDB with AHI > 5 was 72% and with AHI > 20 was 38%. Only 7% of the SDB was primarily central apnea. There was no significant difference in SDB prevalence by event type, timing after stroke, or type of monitoring. Males had a higher percentage of SDB (AHI > 10) than females (65% compared to 48%, P = 0.001). Patients with recurrent strokes had a higher percentage of SDB (AHI > 10) than initial strokes (74% compared to 57%, P = 0.013). Patients with unknown etiology of stroke had a higher and cardioembolic etiology a lower percentage of SDB than patients with other etiologies.51c
From the acute to the subacute phase of stroke, SDB improves.31,35–37,43 Nevertheless, a significant percentage of patients (up to 50%) exhibit an AHI of at least 10/hr months to years after the acute event (see Table 88-1). Central events improve more than obstructive events. One study suggested a better improvement of SDB in hemorrhagic strokes as compared to ischemic strokes.37
Clinical Features
Breathing Disturbances during Sleep
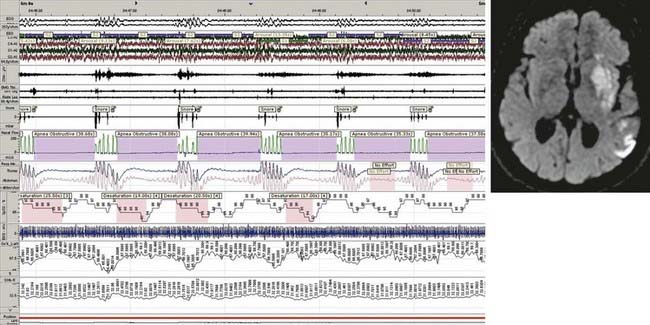
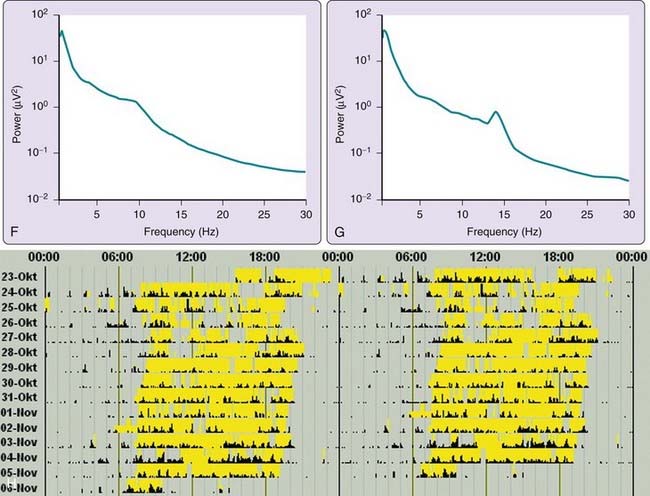
The most common form of SDB in stroke patients is OSA (Fig. 88-1). Severe OSA (with AHI 20 to 30) is found in 20% to 30% of patients. Occasionally, patients present with both OSA and Cheyne-Stokes breathing, with predominance of the first in rapid eye movement (REM) sleep and of the latter in light non-REM (NREM) sleep.26
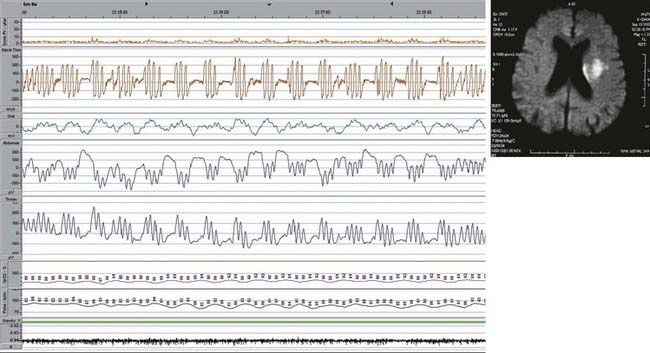
Cheyne-Stokes breathing is a type of periodic breathing in which central apneas and hypopneas are separated by a crescendo–decrescendo respiratory pattern (Fig. 88-2). Central periodic breathing can be defined as three or more cycles of regular crescendo–decrescendo breathing associated with a reduction of at least 50% in nasal airflow and effort lasting at least 10 seconds. In the first few days after stroke, Cheyne-Stokes breathing and central periodic breathing during at least 10% of recording time is present in 10% to 40% of patients31,33,48,52,53
Breathing Disturbances while Awake
Breathing abnormalities occurring during wakefulness following hemispheric strokes have not been well studied. Such abnormalities can include selective impairment of behavioral or volitional respiratory control with preservation of metabolic control mechanisms. Strokes in the frontal cortex, basal ganglia, or capsula interna can cause respiratory apraxia, with impairment of voluntary modulation of breathing amplitude and frequency, leaving patients unable to take a deep breath or hold the breath.54,55
Several abnormal patterns of breathing, though uncommon, can occur after brainstem stroke, especially during sleep or with decreased levels of consciousness. Sustained respiratory rates above 25 to 30/min in the absence of hypoxemia (neurogenic hyperventilation) were originally described in six comatose patients with ventrotegmental pontine strokes,56 but these rates were subsequently attributed to stimulation of lung and chest wall afferent reflexes due to pulmonary congestion.57 Neurogenic hyperventilation after stroke usually indicates a poor prognosis.58,59
Inspiratory breath-holding (apneustic breathing), originally described in two patients with bilateral ventrotegmental mediocaudal (infratrigeminal) pontine stroke,60 is rare and usually secondary to basilar artery occlusion. Erratic variations in breathing frequency and amplitude (ataxic or Biot’s breathing), and failure of automatic breathing (central sleep apnea or Ondine’s curse), usually imply a lateral medullary stroke, often bilateral.61–64 Damage to the medullary reticular formation and nucleus ambiguus may be sufficient to cause a loss of automatic breathing, and a lesion that also includes the nucleus tractus solitarius is necessary to cause failure of both automatic and voluntary respiration.63 Volitional breathing can be impaired by brainstem strokes involving corticobulbar and corticospinal pathways at pontine and medullary levels.55 Repetitive yawning can accompany hypersomnia (e.g., in patients with thalamic stroke) and can also occur as a release phenomenon after extensive corticospinal lesions (e.g., in patients with a locked-in syndrome.65
Spinal cord stroke can impair both automatic and voluntary breathing control.54,66 The topography and extent of the lesion can determine respiratory effects. Anterior spinal artery strokes can affect reticulospinal pathways, located anteriorly in the lateral columns of the first three cervical segments, which are crucial for automatic breathing.66 In contrast, posterior spinal artery strokes can damage corticospinal pathways in the dorsolateral spinal cord and impair voluntary control of breathing.67 Strokes that extend up to the C1 level usually cause severe respiratory insufficiency and necessitate ventilatory support.
Pathophysiology
Sleep-Disordered Breathing as a Risk Factor for Stroke
Several studies have suggested a variety of physiologic mechanisms by which obstructive sleep-disordered breathing could predispose to stroke. Chronically, SDB and even habitual snoring are associated with hypertension, which in turn is an important risk factor for stroke. Findings from the Wisconsin Sleep Cohort have shown that an AHI greater than 15 is independently associated with a threefold increased risk of developing new hypertension within a 4-year period.68 Furthermore, SDB is associated with coronary heart disease, myocardial infarction, heart failure, and atrial fibrillation, all of which can cause stroke.69–71 The consequences of nocturnal respiratory events include intermittent hypoxemia, hypercapnia, sleep fragmentation (occasionally with loss of slow-wave and REM sleep), intrathoracic or intracranial pressure changes, and sympathetic activation. A series of hemodynamic (e.g. blood pressure swings), neural (e.g. sympathetic hyperactivity), metabolic (e.g. insulin resistance), and inflammatory or oxidative changes (e.g., increased levels of C-reactive protein, tumor necrosis factor [TNF]-α, interleukin [IL]-6, adhesion molecules) result, contributing to diabetes, increased platelet aggregation, decreased fibrinolysis, endothelial damage, and atherogenesis.69,72,73 Patients with acute stroke also show an increase in some of the molecular markers and consequences of SDB such as elevated levels of fibrinogen and C-reactive protein.74,75 The observation of an increase in the intima-media thickness of the common carotid artery documented by ultrasound techniques in SDB patients as compared with controls matched for age and vascular risk factors gives further support for an increased (chronic) atherogenesis in SDB.76,77
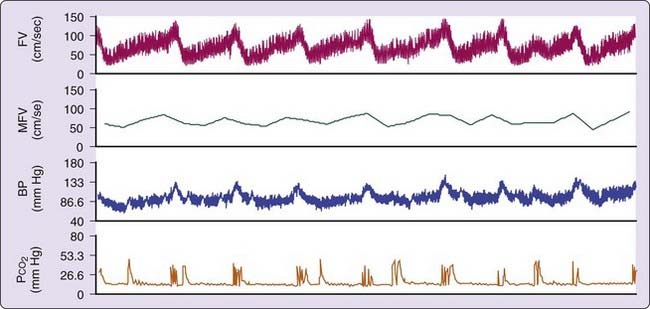
Acutely, apneas and hypopneas during sleep are accompanied by decreased cardiac output, cardiac arrhythmias, systemic hypotension or hypertension, vasodilation due to hypoxia and hypercapnia, and increased intracranial pressure.78 These factors lead to an overall 15% to 20% (in single studies, up to 50%) reduction in cerebral blood flow velocities during respiratory events79–82 (Fig. 88-3). Type, duration, and timing of respiratory events affect hemodynamic consequences. Obstructive apneas of long duration and those that occur during REM sleep may be particularly detrimental.81,83,84 Large fluctuations in cerebral blood velocities (and flow) may be particularly detrimental because patients with SDB have been shown to have a diminished vasodilator reserve.82 Cheyne-Stokes respiration and central apneas also can alter cerebral blood flow.81,85,86 Paradoxical embolization resulting from right-to-left shunting in patients with patent foramen ovale during long apneas is another potential mechanism of stroke.87,88 In view of these observations, it is not surprising that SDB has been associated with the onset of cerebrovascular events at night.32,43 A direct link between SDB and nocturnal cerebrovascular ischemic events has been suggested for retinal infarcts of embolic origin and in single patients with TIA or minor stroke.89–91
A pathogenic role of SDB in vascular disease is further suggested from studies of continuous positive airway pressure (CPAP) treatment. A few studies have shown favorable, although modest, effects of CPAP on mean arterial pressure (MAP).92 Pepperell and coworkers reported that therapeutic CPAP reduced MAP by 2.5 mm Hg, whereas subtherapeutic CPAP levels increased blood pressure by 0.8 mm Hg. Such an effect can lead to a reduction in stroke risk of about 20%.93 Treatment with CPAP can also reverse the detrimental hemodynamic, neural, and molecular effects of SDB-influenced surrogate markers of vascular disease such as factor VII clotting activity, fibrinogen levels, and platelet activation or aggregation.25,72,73,94
Sleep-Disordered Breathing as a Consequence of Stroke
A disturbed coordination of upper airway, intercostal, and diaphragmatic muscles due to brainstem or hemispheric lesions (bulbar and pseudobulbar palsies) might favor the appearance of OSA.95,96 In patients with rostrolateral medullary lesions and SDB, a reduced ventilatory sensitivity to inhaled CO2 can play a role.97 Other factors, including hypoxemia due to aspiration or respiratory infections, reduction of voluntary chest movements on the paralyzed side, supine position, and sleep fragmentation secondary to stroke or stroke complications, can also be involved.45,98
The presence of Cheyne-Stokes breathing has been attributed to CO2-hypersensitivity induced by bilateral supratentorial lesions.99 The presence of bilateral or severe strokes, heart failure, and a decreased level of consciousness, traditionally described in stroke patients with Cheyne-Stokes breathing, is not always necessary.41,48,52,100 Conversely, the damage of specific brain areas (e.g., those belonging to the autonomic network) may play a so-far neglected role, particularly in the acute phase of stroke.48,53 Patients with brainstem stroke can also develop Cheyne-Stokes breathing.52,101–103
Clinical Significance
SDB in patients with cerebrovascular disorders has detrimental effects at different levels. Acutely, SDB detected the first night after brain infarction was found to be associated with early neurologic worsening, longer hospitalization, and higher daytime and nighttime blood pressure levels.32,48,104 Preliminary observations suggest that the acute evolution of stroke, as assessable by magnetic resonance imaging (diffusion and perfusion studies) might also be negatively affected by OSA.105
Chronically, habitual snoring and SDB also affect outcome of stroke. Snoring was found to adversely affects prognosis in stroke survivors.106 Dyken and colleagues reported a 4-year mortality of 21% in stroke patients with SDB.29 Three large subsequent studies confirmed the negative effect of SDB on long-term risk of stroke.43,107,108 Parra’s group reported in 161 patients followed over 23 months that mortality (8%/year) was independently determined by age, initial AHI, coronary heart disease, and involvement of the middle cerebral artery.107 Similarly, Bassetti and colleagues found in 152 patients followed over 60 months that mortality (3%/year) was associated with a higher AHI in the acute phase. Conversely, the incidence of new vascular events (4%/year) was similar in patients with and without SDB.43 In a series of 151 patients observed over 10 years, Sahlin and coworkers found that obstructive—but not central—sleep apnea was a predictor of mortality.108
Several studies have suggested a negative impact on functional outcome. Good and colleagues found a poorer functional outcome in patients with stroke and nocturnal oxygen desaturations.28 Kaneko’s group showed that OSA is significantly and independently related to functional impairment and length of hospitalization in 61 patients admitted to a stroke rehabilitation unit.109 Turkington and colleagues found a relationship between SDB (AHI > 10 found within 24 hours of stroke onset) and functional outcome (as assessed by the Barthel index) and mortality at 6 months in a series of 120 patients.110 In a study of 60 patients, Yan-Fang and Yu-Fing reported a poorer functional outcome (as estimated by the Barthel Index) at 6 months in patients with an AHI greater than 15.47
Diagnosis
Based on its impact on stroke outcome and treatability, SDB should be assessed by history and nocturnal recordings (respirography) in all patients with TIA and stroke. The suspicion should be particularly high in obese men with history of habitual snoring, witnessed apneas, hypertension, diabetes mellitus, and sleep-onset stroke.27,32,43 Stroke topography and etiology and history of excessive daytime sleepiness are, conversely, poor predictors of SDB in stroke victims.
Respirography is sufficiently accurate to diagnose SDB and estimate its severity even in the acute setting.43 Full polysomnography is needed only in a minority of patients. The optimal timing of sleep studies after stroke or TIA is unknown. Although studies within days of a stroke might be less representative of the baseline condition attained several weeks to months after the ischemic event, treatment of SDB soon after stroke could potentially minimize further damage to injured neural tissue and improve outcome.105 Brown and coworkers estimated that screening and treatment of SDB in stroke patients are cost-effective.111
Treatment
Treatment of SDB in stroke patients represents a clinical, technical, and logistical challenge. Treatment strategies should always include prevention and early treatment of secondary complications (e.g., aspiration, respiratory infections, pain) and a cautious use or avoidance of alcohol and sedative-hypnotic drugs, which can all negatively affect breathing control during sleep. Patient positioning in the acute phase can influence oxygen saturation as well.45,98
Continuous positive airway pressure is the treatment of choice for OSA. Automatic CPAP systems can be used for simultaneous detection of upper airway obstructions and treatment, which is made possible by automatic titration of CPAP pressure.34 The compliance for autotitrating continuous positive airway pressure in the first 3 months after TIA was found to be acceptable (≥4 hours per night for ≥75% of nights) in 12 of 30 patients with SDB.51b Compliance is influenced by the spontaneous improvement of SDB after the acute phase (see earlier) and by the absence of excessive daytime sleepiness in most patients with stroke and SDB. In addition, compliance can be expected to be a problem also in stroke patients with dementia, delirium, aphasia, anosognosia, and pseudobulbar or bulbar palsy.
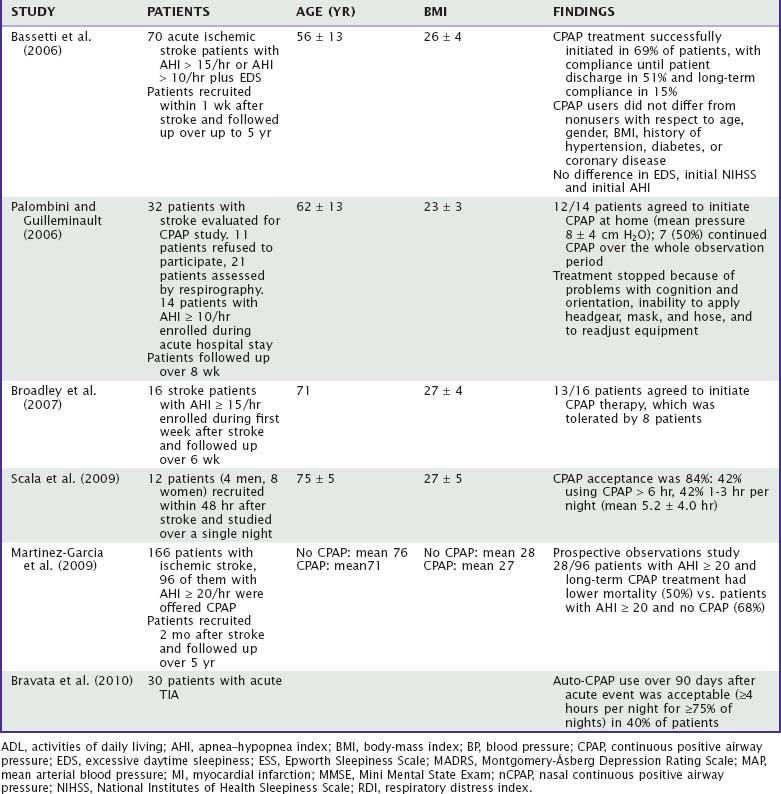
Treatment of SDB in stroke patients was assessed in 10 studies and almost 600 patients (Table 88-2). Wessendorf and colleagues reported a 70% CPAP compliance in 105 stroke patients admitted in a rehabilitation unit. Poor compliance was associated with aphasia and severe stroke. CPAP use led to an improvement of subjective well-being and nighttime blood pressure values in a group of 41 and 16 patients, respectively.30 Sandberg’s group reported a reduction in depressive symptoms after one month of CPAP and poor compliance in patients with delirium and cognitive deficits. No improvement was conversely found on neurologic recovery, as assessed by the Barthel index.112 Disler and colleagues reported good compliance in five consecutive patients with stroke and SDB treated in a rehabilitation unit.34
Later studies found a generally lower CPAP compliance (about 50% in the acute phase and 30% in the chronic phase; range, 12% to 82%) in stroke patients with SDB. Hui and coworkers found that only 4 (12%) out of 34 patients continued CPAP over more than 3 months.35 Martinez-Garcia and colleagues reported in a series of 51 patients a compliance of 29% over the first month.50 In that study, CPAP use led to a significant, fivefold decrease in vascular events. Bassetti’s group reported that only 8 (22%) out of 36 patients discharged from acute hospital with CPAP continued this treatment on a long-term base (60 months).43 No predictors of long-term compliance could be found. Palombini and colleagues found a compliance of only 22% over the first 2 months after stroke.113 Hsu and coworkers randomized 30 patients with stroke and AHI of 30 to CPAP or no treatment.114 Only 7 (47%) of 15 patients kept CPAP for more than 4 weeks and the average use of CPAP was only 1.4 hours per night.
Martinez and colleagues reported a reduction of 5-year mortality in 28 stroke patients with mild to moderate SDB (AHI = 20) who were treated with CPAP as compared to 68 patients with SDB who did not tolerate treatment.49 In this study, the long-term CPAP compliance was 30%. The choice of the headgear may have had an influence on the acceptance of CPAP.115
In patients with central apneas and Cheyne-Stokes breathing improvements of breathing disturbances may be achieved with oxygen.102 A method of ventilatory support called adaptive servo-ventilation may also be considered. Wessendorf’s group found that adaptive servoventilation prevented central apneas in stroke patients with heart failure more efficiently than CPAP or oxygen.116
Tracheostomy and mechanical ventilation might become necessary in patients with central hypoventilation. Control of central apneas and ataxic breathing usually requires assisted ventilation. Hiccup can be treated with neuroleptics or with baclofen.117
Sleep–Wake Disturbances
Epidemiology
In a series of 285 consecutive patients we found that 21 months after stroke 27% of patients slept at least 10 hours per day (hypersomnia), 28% had an Epworth Sleepiness Scale score of at least 10 (excessive daytime sleepiness), and 46% had a fatigue severity scale score of at least 3 (fatigue) (unpublished observation). Other studies have similarly suggested a high frequency (30% to 70% of patients) of poststroke fatigue.118,119
In a series of 277 consecutive patients evaluated 3 months after stroke, insomnia was reported by 57% of patients. In 18% of the 277 patients, insomnia appeared de novo after stroke.120
Sleep-related movement disorders and parasomnias are less often observed after stroke. In a series of 137 patients assessed 1 month after stroke, restless legs symptom (RLS) symptoms were found de novo in 12% of patients.121
Other sleep–wake disturbances following stroke include an abnormal transition from wake to sleep and vice versa, with confusion between dream and reality (oneiric state), dream changes, and an altered perception of time (Zeitgefühl).122,123
Clinical Features
Hypersomnia and Excessive Daytime Sleepiness
Hypersomnia is defined clinically as a reduced latency to sleep, increased sleep, or excessive daytime sleepiness. In patients with strokes that affect activating arousal pathways or the paramedian thalamus, hypersomnia can alternate with insomnia (see earlier). Façon and coworkers described, for example, a 78-year-old patient with a tegmental mesencephalic infarct in whom severe, persistent hypersomnia was accompanied by an inversion of the sleep–wake cycle with nocturnal agitation.124
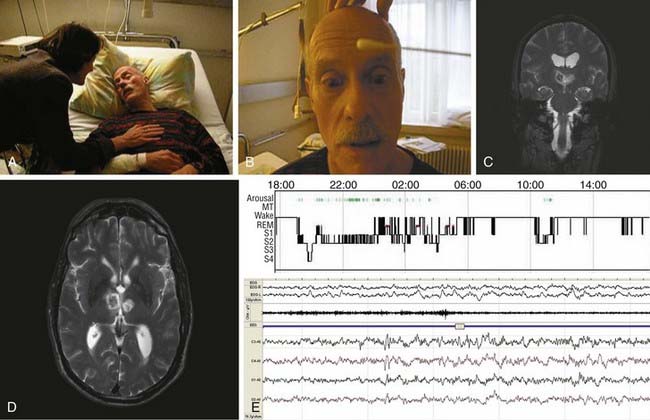
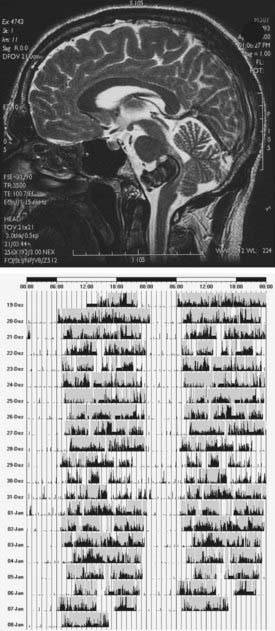
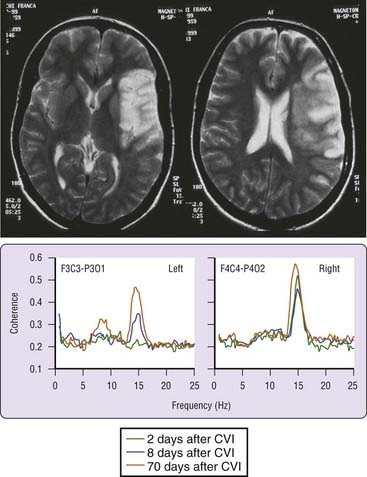
Poststroke hypersomnia, with or without excessive daytime sleepiness, is usually observed in association with thalamic (Fig. 88-4), mesencephalic, or upper pontine strokes. Less commonly, strokes in the caudate, striatum, lower pons (Fig. 88-5), medial medulla, and cerebral hemispheres (with or without mass effect) (Fig. 88-6) may be complicated by hypersomnia.125
In deep (subcortical) hemispheric and thalamic stroke, hypersomnia may correspond to presleep behavior, during which patients yawn, stretch, close their eyes, curl up, and assume a normal sleeping posture, while complaining of a constant sleep urge.126 Some of these patients are able to control this behavior when stimulated or given explicit, active tasks to perform. This presleep behavior may be compulsive in that removing the patient from bed can result in repeated attempts to lie down and adopt a sleeping posture. However, during what appear to be daytime sleep periods, relatively quick responses to questions or requests suggest wakefulness. For this peculiar dissociation between lack of autoactivation in the presence of preserved heteroactivation, Laplane suggested the term athymormia, or “pure psychic akinesia.”127
In some patients, hypersomnia evolves to extreme apathy, with lack of spontaneity and initiative, slowness, poverty of movement, and catalepsy, a condition for which the term akinetic mutism was coined.128 Akinetic mutism, and a less severe form, usually referred to as abulia, can persist despite normalization of vigilance or even after appearance of insomnia. In some of these patients, the eventual diagnosis is poststroke fatigue (see later) or post-stroke depression.
Narcolepsy-like phenotypes have rarely been seen following stroke, even in the absence of human leukocyte antigen (HLA) positivity and cerebrospinal fluid (CSF) hypocretin-1 deficiency.129–131 Hypersomnia with hyperphagia (Kleine-Levin–like syndrome) was reported after multiple cerebral strokes.132
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree