Chapter 99 Sleep Bruxism
Abstract
The most recent International Classification of Sleep Disorders1 categorizes sleep bruxism as a sleep-related movement disorder and defines it as an oral activity characterized by grinding or clenching of the teeth during sleep. Because it is probable that sleep bruxism differs in terms of etiology from daytime parafunctional jaw muscle activity,2 it should be distinguished from teeth clenching, bracing, or gnashing while awake.3
Teeth grinding and sleep bruxism frequency is reportedly variable over time; some patients go several nights or even weeks without bruxism episodes.2 However, in cases of moderate to severe sleep bruxism, teeth grinding is present every week.4 Patients with sleep bruxism typically experience either phasic (rhythmic) or tonic (sustained) motor activity in jaw muscles (e.g., the masseter and temporalis), with the variable presence of teeth grinding sounds.2,5 The element of sound is the major reason people seek consultation. Teeth-grinding sounds disrupt the bed partner’s sleep. Other reasons for seeking help are related to tooth wear, orofacial pain or temporal area headaches, and tooth hypersensitivity to cold air, beverages, and food.
An absence of a medical cause is considered to be primary, or idiopathic sleep bruxism, whereas sleep bruxism associated with a medical condition is the secondary form (Boxes 99-1 and 99-2). The latter is noted after drug intake or withdrawal (e.g., neuroleptics that can induce oral tardive dyskinesia and grinding); this secondary form is classified as iatrogenic. Several orofacial activities are often concomitant: grimacing, chewing automatism, or excessive lip and tongue movements such as thrust or protrusion. In this chapter, the term sleep bruxism is used to denote teeth grinding and oromotor-related activities occurring during sleep, as reported by the bed partner or family, regardless of whether it occurs in the primary or secondary (iatrogenic) form.
Box 99-1 Types of Bruxism
Box 99-2 Secondary or Iatrogenic Bruxism
The following medical conditions and drug chemicals have been associated with or reported in literature with teeth grinding or bruxism-like orofacial motor activities.2,15,19,30,42–44,47,48,56,70–73,86–88,90 Not all associated bruxism is sleep related.
Etiology
Throughout the 20th century, support for different theories regarding the etiology of bruxism has swung like a pendulum; theories range from the role of peripheral dental occlusion,6 to a global explanation that includes personality style, the person’s capacity to adapt to life pressures presented by the psychosocial environment, and neurochemical and homeostatic sleep maintenance versus arousal mechanisms associated with activity of the autonomic and motor nervous systems.2,6–11
Epidemiology and Risk Factors
Most studies and surveys reporting the prevalence (the number of positive cases at a given time) of sleep bruxism are based on self-reports of clenching during the daytime or both clenching and grinding during sleep. As yet, no longitudinal study using biological recordings has been conducted to estimate the fluctuation or persistence of sleep bruxism in a given person with respect to age. Moreover, many patients with sleep bruxism are not aware of grinding if they sleep alone or with a bed partner who sleeps deeply. The overall prevalence of awake clenching is approximately 20% of the adult population, with more women reporting their awareness of clenching than men.12 According to parents’ reports, the incidence of sleep grinding in children younger than 11 years is between 14% and 20%. Concomitant oral activity such as nail biting (onycophagia) is also noted in 9% to 28% of those reporting sleep bruxism, thumb sucking is seen in 21% of children, and snoring is heard in 14%.13,14 In adults, the prevalence of grinding drops with age, from 13% in those 18 to 29 years old to 3% in those 60 years and older, with a mean of 8%.15 No further gender differences have been noted. Caution is necessary in interpreting these numbers, however, because reduced incidence with age can result from inaccurate estimates due to the high prevalence of denture users in the older population (e.g., greater than 40% in some geographic areas).
Several concomitant risk factors have been linked to sleep bruxism. Psychological factors such as anxiety, competitiveness, stress, and maladaptive or less-positive coping strategies16 have been associated with sleep bruxism, but some findings remain controversial.17,18 Restless legs syndrome is associated with sleep bruxism, but only in 10% of the RLS population. Survey results show sleep apnea to be a risk factor (significant low odds ratio), a finding to further validate.19,19a Regarding smoking as a risk factor, it remains to be determined whether the variance can be explained by the physiologic influences of nicotine or the perception of smoking as an oral habit.20 Another variable is the concomitant finding of orofacial pain or joint sound or lock at the temporomandibular level; patients with sleep bruxism are more at risk for pain and jaw limitation.21,22 For example, we found that half of patients with high incidence of sleep bruxism muscle activity reported mild pain in the morning, which was associated with a lower frequency of sleep bruxism episodes per hour of sleep than in matched controls.22–24 The prevalence of grinding noted in an institutionalized developmentally delayed population is similar to the figure reported in the general population.15,25 A study made of 5- to 18-year-old children with sleep bruxism, as confirmed in sleep laboratory, revealed a higher prevalence of attention behavior and somatic problems.26 Use of several medications or recreational drugs is also a risk factor for sleep bruxism (see Box 99-2).
Pathophysiology
No single mechanism or theory explains the pathophysiology of sleep bruxism.7,23,27 Rather, this motor activity is more likely to be the product of biological and psychosocial influences in a given person (Video 99-1![]() ). Bruxism probably results from a series of influences that do not obey a mechanistic model or the logic of an algorithm.
). Bruxism probably results from a series of influences that do not obey a mechanistic model or the logic of an algorithm.
Rhythmical Masticatory Muscle Activity during Sleep in Asymptomatic Sleepers
Research involving the recording of masseter electromyographic EMG activity to assess rhythmic masticatory motor activity (RMMA) in the jaw-closer muscles found that about 60% of “normal” sleepers exhibited RMMA (three masseter muscle bursts or contractions within an episode, in the absence of teeth grinding) during sleep.28 In patients with somnambulism and sleep terrors, the term chewing automatism was used to describe the slow rhythmic masticatory activity.29 This term was later used in the identification of the orofacial activities noticed with the rapid eye movement (REM) behavior disorders.30 Phasic oromotor activity is associated with RMMA, and in sleep bruxism it is probably an extreme manifestation of an ongoing or natural activity during sleep.
Stress and Psychosocial Influences
Bruxism was associated with anxiety and hyperactivity, but strong evidence is absent.27 Rigorous evidence is lacking to support the notion that sleep bruxism is an anxiety-related disorder.17,19 However, patients with sleep bruxism seem to be more task-oriented as a result of their personality and coping style, as opposed to being in a pathologic stress or anxiety-related pattern.
Findings in Sleep
Studies comparing young, healthy patients who have sleep bruxism and control subjects have shown that the former demonstrate normal sleep organization and macrostructure.5,31–33 Their sleep latency, total sleep time, percentage of time spent in the various sleep stages, and number of awakenings are within normal limits. They also report a normal amount of time spent awake during the sleep period, and they demonstrate sleep efficiency that falls within the usual range of good sleepers (greater than 90%). Thus, they do not complain about sleeping poorly.32 Interestingly, and like episodes of sleep apnea in patients with that disorder, most sleep bruxism episodes (74%) were observed while sleepers were in the supine position.34
Sleep bruxism episodes are most often (greater than 80% of the time) scored in sleep stages 1 and 2,26,32,33,35 although the literature is confusing on this point.36 A high incidence of destructive sleep bruxism, scored from electroencephalographic (EEG) artifacts, was also previously reported in REM sleep36 in patients suffering from depression, and this probably represented secondary sleep bruxism. In sleep bruxism subjects, it was observed that approximately 10% to 25% of sleep bruxism occurred in REM.26,34,35
Most studies support the idea that rather than triggering sleep arousal, sleep bruxism is concomitant or secondary to cyclic alteration in sleep patterns. During sleep, every 20 to 60 seconds, an observable cyclic electroencephalographic-electrocardiographic-electromyographic (EEG-ECG-EMG) activation occurs, called the cyclic alternating pattern (CAP). Interestingly, close to 80% of sleep bruxism episodes have been observed in association with the CAP, which may act as a resetting mechanism for physiologic functions in relation to sleep environment or endogenous factors.7,9,27,33 The association between sleep bruxism and CAP is supported by findings showing that more than 50% of sleep bruxism episodes occur in clusters (within 100 seconds) and that approximately 15% to 20% occur in the transition from deep sleep (stage 3-4) to REM sleep.33,37 These findings are also consistent with the observation that sleep bruxism is preceded by alpha EEG activity and is associated with a tachycardia.35,38
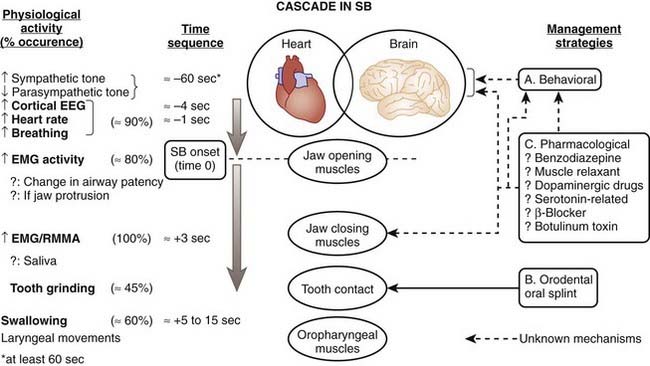
We have also noticed that RMMA or sleep bruxism episodes occur after physiologic events. In the minutes preceding RMMA or sleep bruxism episodes, there is a slight change in autonomic-cardiac sympathetic (increased) and parasympathetic (decreased) balance; then, at minus 4 seconds, there is a rise in EEG alpha and delta activity, followed by a tachycardia initiated one heartbeat before the onset of jaw-opener muscle activity, associated with a major rise in respiratory amplitude (big breath). About 800 msec later, the jaw-closer muscle contractions start.8,9,27,37,38 These findings support the concept that sleep bruxism is secondary to exaggerated transient motor and autonomic nervous system activation in relation to sleep arousals. Also, young and otherwise healthy patients with sleep bruxism show a normal rate of brief arousals (less than 14 per hour of sleep).35,39
To further explore the mechanism involved in initiating sudden RMMA or sleep bruxism, we developed an experimental model that could trigger arousals during sleep without inducing awakenings. The application of a brief vibrotactile stimulation induced frequent sleep arousals that were followed by RMMA in all patients with sleep bruxism, with teeth-grinding occurring in 86% of trials.40 We therefore suggest that sleep bruxism or teeth grinding is one of the events occurring along the sequence of physiologic activations associated with microarousals (Fig. 99-1).7,9,27,38
The role of cortical EEG activity in the genesis of sleep bruxism has also been studied. There is an increase in alpha and delta activity in the cascade of physiologic activations associated with microarousals.38 EEG K-complexes were not reported to be more frequent in the 60 seconds before sleep bruxism episodes, and, overall, patients with sleep bruxism had 40% fewer K-complex events than matched normals.41 This finding is contrary to previous reports based on studies made in the absence of comparison with normal sleepers. Indirect evidence further suggests that trigeminal motor excitability in patients with self-reported teeth grinding comes mainly under brainstem and not cortical influences.7,9,27
Oromotor Excitability
There is little experimental evidence to support a role for the motor system as a primary factor in the genesis of sleep bruxism. At best, some very indirect information from animal physiologic studies may help us understand how the motor system is comodulated during sleep.7 For humans, as for animals, it has been suggested that fluctuations in the activity of the reticular motor area in relation to sleep onset and maintenance could be associated with brief periodic motor excitation.9,27
Catecholamines and Neurochemistry
An early report on Parkinson’s disease linking the use of levodopa (L-dopa) to teeth grinding provided initial support for the suggestion that grinding may be related to dopaminergic brain-related neurotransmitters.2 Teeth grinding, as a manifestation of oromandibular tardive dyskinesia, was also reported for some schizophrenic patients being treated with neuroleptic drugs.42 From these reports, the association of dopamine with sleep bruxism pathophysiology appeared somewhat weak. These patients already had altered nigrostriatal neurons due to disease or to medication.
Some evidence further suggests that catecholamines, such as dopamine and norepinephrine, might have a role in sleep bruxism pathophysiology.11,43,44 A controlled polysomnographic study of patients with sleep bruxism with the catecholamine precursor L-dopa revealed a modest reduction in sleep bruxism frequency in comparison to placebo.43 The use of a dopaminergic modest agonist, bromocriptine, in a double-blind control design (using domperidone, a peripheral dopamine blocker, to prevent side effects such as nausea or vomiting) failed to show either a reduction in sleep bruxism motor episodes or a change in dopamine striatal binding.44
The other catecholamine-related medication reported to reduce sleep bruxism and teeth grinding is propranolol, a beta-blocker.45 This medication was also associated with a reduction in daytime teeth grinding in two neuroleptic-treated patients presenting with abnormal orofacial movements.46 However, a controlled study of young patients with sleep bruxism revealed that propranolol was not deleterious to their sleep and neither reduced teeth grinding nor influenced the frequency and duration of jaw muscle contraction.47 Also, when interpreting studies using specific pharmacologic agents in sleep bruxism, it should be noted that other neurotransmitters (e.g., serotonin, cholecystokinin, gamma-aminobutyric acid) and drugs (e.g., selective serotonin reuptake inhibitors, dopamine antagonists, calcium channel inhibitors) exacerbate teeth grinding and rhythmic movement modulation (see Box 99-2).7,9,27,48
Genetics and Familial Predisposition
As yet, no genetic markers have been found for transmitting sleep bruxism. It is, however, interesting that between 21% and 50% of patients with sleep bruxism have a first-degree relative who ground his or her teeth in childhood.13,49 Furthermore, studies of monozygotic and dizygotic twins showed that sleep bruxism was more often observed in monozygotic twins.49,50 A concomitant finding in a recent Finnish study revealed that childhood sleep bruxism persisted in a large number of adults (greater than 86.9%).50 On the other hand, a twin study did not find any genetic correlation with sleep bruxism.51 Thus, the pattern of inheritance for sleep bruxism is unknown, and the role and influences of familial and environmental factors remain to be assessed.27,50
Possible Role of Local Factors
It has been suggested that tooth occlusal or morphologic interferences were responsible for teeth grinding. Although this concept of a peripheral cause for sleep bruxism is still reported in the literature, it remains controversial.6,27,52–54 On the basis of noncontrolled evidence, tooth contact is not a dominant activity in a 24-hour cycle; it has been estimated to occur for approximately 17.5 minutes per 24 hours.55 Moreover, during sleep, oromotor muscle activity related to sleep bruxism is present for approximately 8 minutes (about 2% of sleep time) over a 7- to 8-hour sleep period and does not always occur with tooth contact; grinding sounds are reported in approximately 44% of sleep bruxism or RMMA episodes.5,34 The debate over the role of dental occlusion in sleep bruxism is beyond the scope of this chapter; reviews have described peripheral sensory influences on sleep bruxism.52,53
Reduced Salivary Flow, Airway Patency, and Jaw Motor Activity during Sleep
Another question is whether the lower salivary flow during sleep increases the risk for tooth wear.56 It appears that no sleep data directly support this premise. The low salivary flow observed during sleep may be related to low swallowing frequency: between 2.1 and 9.1 swallowing movements per hour are observed in sleep, compared with 25 per hour during daytime.57 Based on data retrieved from an analysis of laryngeal swallowing movements observed in controls and in sleep-bruxism subjects, it was hypothesized that RMMA could be associated with an increase in salivary flow during sleep to lubricate the oroesophageal tissues.34,56 Lack of saliva, a natural orodental lubricant, may then cause a dramatic breakdown in the tooth structure of patients with sleep bruxism. Consequently, specific clinical management could be planned for oral dryness in sleep.56 However, it remains to be understood why some frequent teeth grinders show little tooth wear; it may be that a better quality and volume of saliva lubrication or stronger tooth enamel (e.g., density) explains such observations.
Sleep breathing disorders (e.g., sleep apnea) have been associated with sleep bruxism.19,19a Results from a large population survey have suggested that self-reports of sleep apnea were two or three times higher in subjects also aware of teeth grinding.19 Because sleep is usually associated with a jaw-opening retrusive position, a tongue muscle relaxation (e.g., genioglossus), and a reduction of airway patency, it remains to be investigated whether RMMA-related sleep arousal assists in the recovery of airway patency or whether this is just a concomitant and typical motor response associated with sleep arousal.7–927
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree