Organization of Sleep Stages
Rapid eye movement (REM) sleep, sometimes called dreaming sleep, and non-REM (NREM) sleep are the two sleep states. REM sleep alternates with NREM in recurring cycles of approximately 90 minutes. Recordings derived from electroencephalography (EEG), eye movements (electro-oculograms [EOGs]), and surface electromyography (EMG) of muscles (typically chin) are necessary to identify sleep states. NREM sleep has classically been divided into four stages (1 to 4), which represent progressive deepening of sleep. A recent revision of staging nomenclature now classifies these stages as N1, N2, and N3 (
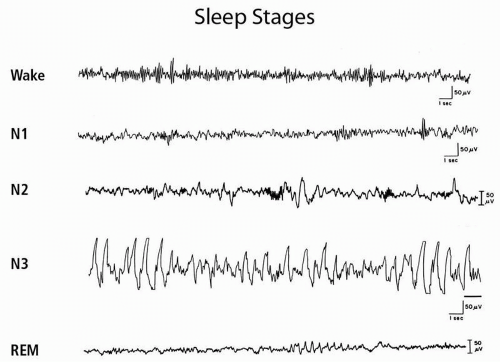
Fig. 21.1). The waking EEG in quiet wakefulness shows the characteristic alpha rhythm, a posterior predominant 8- to 13-Hz rhythm that attenuates with eye opening. Stage N1 (stage 1) is characterized by the gradual disappearance of alpha rhythms, which are replaced by 4- to 7-Hz theta rhythms and some faster activity. The emergence of sleep spindles (11- to 16-Hz sinusoidal transients lasting at least 0.5 seconds) and K complexes (negative sharp wave followed by positive component lasting ≥0.5 seconds) defines N2 (stage 2). Stages 3 and 4 are now grouped together under the term N3. N3 is characterized by high-voltage slow wave activity with frequencies of 0.5 to 2 Hz. Stage N3 is often referred to as delta sleep, slow wave sleep, or deep sleep. N3 can be characterized as “deep sleep” because subjects are difficult to arouse and typically amnestic for events that occur on arousal from this sleep stage. Although detailed narrative dreaming does not seem to occur, subjects awoken from N3 may report fragmentary dream mentation or that they were “thinking” (see parasomnias below). The normal young adult descends in an orderly progression through the three NREM stages. N3 appears approximately 30 to 40 minutes after sleep onset. The first REM period (stage R) follows this slow wave sleep, approximately 70 to 90 minutes after sleep onset.
The PSG during REM sleep shows dramatic changes. A sudden loss of EMG activity occurs in the chin muscles, which is indicative of generalized skeletal muscle atonia. Rapid eye movements occur in phasic bursts, and the EEG shows mixed frequencies similar to those in waking and stage 1 sleep, sometimes with a characteristic “saw tooth” pattern.
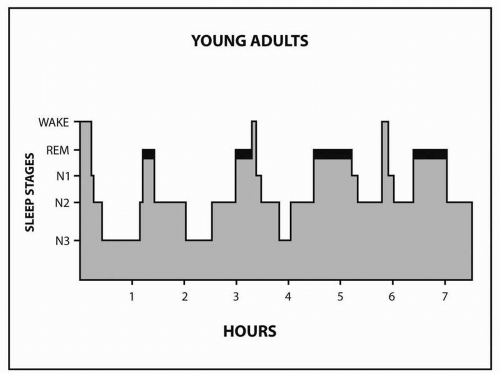
The first REM period is short, lasting about 10 minutes. The end of the first REM period completes the first sleep cycle. Thereafter, NREM sleep continues to alternate with REM sleep; the healthy adult goes through 4 to 6 cycles (
Fig. 21.2).
Sleep architecture is the organization of sleep stages and cycles. The normal young adult spends approximately 5% of the night in stage N1, 50% to 55% in stage N2, 20% in N3, and 20% to 25% in REM. N3 is concentrated in the first third of the night, whereas REM episodes become progressively longer later in the night. N3 decreases as a function of age, whereas REM remains fairly constant after early childhood. Of the newborn’s daily 17 to 18 hours of sleep, 50% is REM. Children and early adolescents sleep 10 to 11 hours. Most adults prefer to sleep 7.5 to 8.5 hours. Subjects sleep-restricted to 6 hours show cognitive deficits that are cumulative. Deprivation of REM sleep by medication, sleep disruption, or sleep deprivation results in REM rebound when the cause of the deprivation is removed. Sleep deprivation also induces N3 rebound sleep during recovery sleep.
REM and NREM sleep differ physiologically. REM sleep is characterized by both phasic and tonic changes in physiology. The drop in baseline EMG correlates with a tonic change. Rapid eye movements correlate with phasic changes. Tonic physiological changes also include impaired thermoregulation, reduction in ventilatory chemosensitivity, hypotension, bradycardia, increased cerebral blood flow and intracranial pressure, increased respiratory rate, and penile erection. Phasic changes include vasoconstriction, increased blood pressure, tachycardia, and further increases in cerebral blood flow and respiratory rate. During NREM sleep, the physiological state is more stable, with an overall reduction in blood pressure, heart rate, cardiac output, and ventilation. One characteristic feature of N3 is the secretion of growth hormone.
Some disorders are exacerbated by or occur only during certain sleep stages. Sleepwalking, for example, occurs with arousal from NREM, usually N3 sleep. Epileptic seizures tend to be facilitated by NREM sleep but inhibited by REM sleep. Obstructive sleep apnea is typically worse in REM sleep because of REM atonia and decreased respiratory chemosensitivity.



 non-REM slow eye movements
non-REM slow eye movements
 ⇔
⇔


 ⇔
⇔
 Spindle activity and stage 2
Spindle activity and stage 2



 ⇔
⇔
 respiratory drive
respiratory drive
 SWS
SWS
 SWS and REM
SWS and REM