Chapter 138 Sleep Disturbances and Sleep-Related Disorders in Pregnancy
Abstract
Complaints of sleep disturbances are common during pregnancy, generally commencing with the onset of pregnancy, and increasing in frequency and duration as pregnancy progresses due to pregnancy-related anatomic, physiologic and hormonal changes.1–3 These changes can also cause sleep disorders or exacerbate prepregnancy sleep disorders, both of which can threaten maternal (mental and physical) health and fetal well-being.4–7
Pregnancy-Induced Hormonal Changes and Their Effect on Sleep
Pregnancy brings about significant changes in the maternal endocrine milieu including the gonadal steroids (estrogen and progesterone), pituitary hormones (gonadatropins, prolactin, growth hormone), melatonin, and cortisol.8–10 These hormonal changes not only affect the sleep–wake cycle and sleep structure directly, but they also cause physiologic changes that lead to sleep disturbances (detailed in the later discussion of physiologic changes).
The main sex steroids progesterone and estrogen show a 24-hour rhythm at 35 weeks’ gestation. Serum progesterone has a diurnal change, with higher concentrations in the evening.11 The maternal plasma estriol has a phase that is opposite to that of the cortisol rhythm, which is discussed later.9
Progesterone
Progesterone significantly increases across pregnancy, with levels 10 to 5000 times higher at term compared to the nonpregnant phase and stage of ovarian cycle.8 Complaints of sleepiness and fatigue may be attributable to increased progesterone during pregnancy.12 Progesterone deactivates the brain and produces soporific and sedating effects by activating intracellular progesterone receptors and its metabolites, targeting brain gamma-aminobutyric acid (GABA) receptors.10,13,14 Animal and human studies have demonstrated that progesterone administration decreases the amount of wakefulness and rapid eye movement (REM) sleep and reduces the latency to non-REM (NREM) sleep.10,13–15 On the other hand, progesterone-induced smooth muscle relaxation can contribute to frequent urination, heartburn, and pregnancy rhinitis, causing nocturnal awakenings.6,13,14,16–18 The increase in levels of progesterone is also believed to raise the body temperature that disrupts sleep quality.19
Estrogen
The estrogen level during late pregnancy is approximately 1000 times higher than in the ovulatory premenopausal period.8 Unlike progesterone, estrogen has been reported to have excitatory effects in the nervous system and to selectively decrease REM sleep activation of sleep-active neurons in the ventrolateral preoptic area.20 Increased estrogen concentrations during pregnancy cause vasodilation,8 which can result in edema in the extremities and nasal obstruction.8,18
Cortisol
Cortisol starts to increase from the 25th to the 28th week; the most dramatic increase is seen in late pregnancy. Cortisol rapidly returns to normal concentrations after delivery.11
Cortisol has a complex circadian rhythm. It decreases to the lowest level in the early hours of night and increases at the time of morning awakening, reaching its peak at noon.9,11 In pregnant women, this peak is not obvious, probably due to the blunting effect of placental corticotropin (ACTH) on maternal cortisol concentrations.9–11
A human study of cortisol infusions showed decrements in REM sleep and increases in slow-wave sleep (SWS),10,21 probably due to increased progesterone, which elevates the free cortisol.9 Pregnant women with poor sleep in the third trimester have lower cortisol-to-melatonin ratios than good sleepers as a result of a lower early morning peak in their cortisol levels and a relatively higher concentration of melatonin.14
Melatonin
Concentrations of serum melatonin in healthy women display a clear diurnal rhythm in both early and late pregnancy.14,21 However, the circadian melatonin concentration rhythm is lost in preeclamptic women, who have altered blood pressure rhythm.21 Altered rhythm or low levels of melatonin secretion might lead to some pregnancy complications. Serum melatonin levels have been found to be significantly higher during pregnancy and labor than during the postpartum period.21 In singleton pregnancies, nighttime serum melatonin levels steadily increased after 24 weeks of gestation and exhibited significantly higher levels after 32 weeks and around the period of delivery, but they decreased to nonpregnant levels on the second day after delivery.21 In twin pregnancies nighttime melatonin levels were significantly higher after 28 weeks of gestation than normal singleton pregnancies.21
Prolactin
Plasma levels of prolactin increase tenfold in pregnant women compared with nonpregnant women.8 Studies showed that SWS is enhanced by prolactin secretion.10,22 Increased maternal SWS has been reported in lactating women compared to nonlactating women, probably due to increased prolactin level, although hormone levels were not measured.22
The type of delivery affects prolactin secretion. The basal level of prolactin rapidly decreases during active labor and reaches its highest level again up to 6 hours after vaginal delivery. This dramatic fluctuation in prolactin is not seen in elective cesarean births.23
Oxytocin
Oxytocin has been reported to have a rhythm in late pregnancy, reaching the highest level at night together with the phase of the uterine activity rhythm.9 The rhythm of nocturnal uterine activity can compromise maternal and fetal blood flow and oxygen delivery,14 and contribute to nocturnal awakenings during the third trimester of pregnancy.1–3 The increased incidence of labor and delivery during the evening hours can result from increased maternal plasma oxytocin concentrations at night9,21 and cause significant sleep loss.
Growth Hormone
The secretion of growth hormone, essential for tissue growth and protein anabolism, is prompted by ghrelin and growth hormone–releasing hormone (GHRH).10 These hormones are closely associated with the onset and maintenance of SWS and play significant roles in sleep regulation.10,22 This suggests that growth hormone secretion and maintenance of SWS are important for fetal development and the mother’s health during pregnancy.
Relaxin
Relaxin (primarily secreted by the corpus luteum during pregnancy) increases tenfold during pregnancy. It is associated with remodeling of the connective tissue fibers and inhibiting smooth muscle contractility to prepare the uterus for delivery.8,9 These relaxin-related changes can contribute to sleep-disordered breathing (SDB) due to relaxation of airway muscle, carpal tunnel syndrome due to fluid retention within connective tissue, and low back pain due to ligamentous laxity.8 However, the precise mechanisms for these problems associated with relaxin remain to be clarified.
Physiologic Changes in Pregnancy That Could Contribute to Sleep Problems
In addition to the hormonal changes, pregnancy causes a multitude of anatomic and physiologic changes (Fig. 138-1).8,14,16,17,24,25 These changes are essential to maintain pregnancy, but they can contribute to sleep problems during pregnancy.
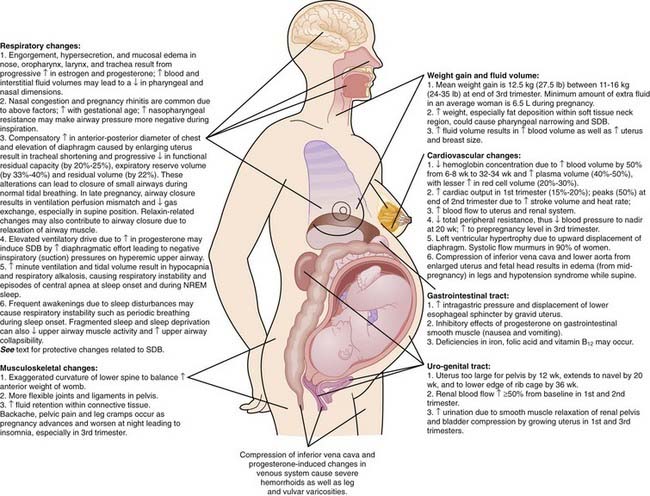
Figure 138-1 Pregnancy-related changes that can affect sleep.
(Modified from American Medical Association, Atlas of the body: changes during pregnancy. Available at: http://www.medem.com/medlb/article_detaillb.cfm?article_ID=ZZZO0MM56JC&sub_cat=516. Requires paid subscription. Accessed August 10, 2008.)
Common Sleep Complaints During Pregnancy
First Trimester
Fatigue is one of the first symptoms of pregnancy associated with prepregnancy levels of iron, ferritin, and hemoglobin and with younger age.12 Similarly, daytime sleepiness is prevalent (37.5% at 6 to 7 weeks of pregnancy), which is attributable to the somnogenic effect of progesterone and fragmented sleep.3,5,10,13,15,26 Sleep disturbances increase 1.4-fold relative to the prepregnancy period owing to nausea and vomiting, urinary frequency (51%), backache and other physical discomforts (e.g., tenderness and a tingling feeling in the breasts) and altered appetite caused by hormonal changes.1,3,8,27 Elevated anxiety, especially in the case of unplanned pregnancies or the absence of psychosocial support in first-time pregnancies, is common.1,28
Second Trimester
Women acclimate to hormonal changes. Sleep disturbances, such as nausea and frequent urination (28.2%) (due to the growing fetus moving above the bladder), also decrease. As a result, energy levels increase and daytime sleepiness decreases.1–3,5,12 However, at the end of the second trimester, irregular uterus contractions (Braxton-Hicks), fetal movements, heartburn, and snoring start to disrupt sleep.2,3,8,26
Third Trimester
The majority of women typically report restless sleep (30.3%) and multiple nocturnal awakenings (98%) due to the continuation of symptoms that started at the end of the second trimester: frequent urination (47% to 95%),1–329 leg cramps, shortness of breath, heartburn, forced body position in bed, backache, joint pain, carpal tunnel symptoms (e.g., numbness), breast tenderness, and itching.1–3,29–31 Women also attribute their sleep loss to internal factors (vivid dreams or nightmares associated with anxiety about labor and delivery, fetus, pregnancy complications, worry about their sleep loss)1,3,5,29,32,33 and external factors such as their children and environmental noise.1–329 The incidence of sleep-related disorders such as SDB and restless legs syndrome (RLS) and associated complications increase (discussed later). Daytime sleepiness (up to 65%) and fatigue, associated with less total sleep and low folic acid, have been reported to increase.1–3,5,27,29,34,35
Changes in Sleep Architecture and Sleep Quality in Normal Pregnancy
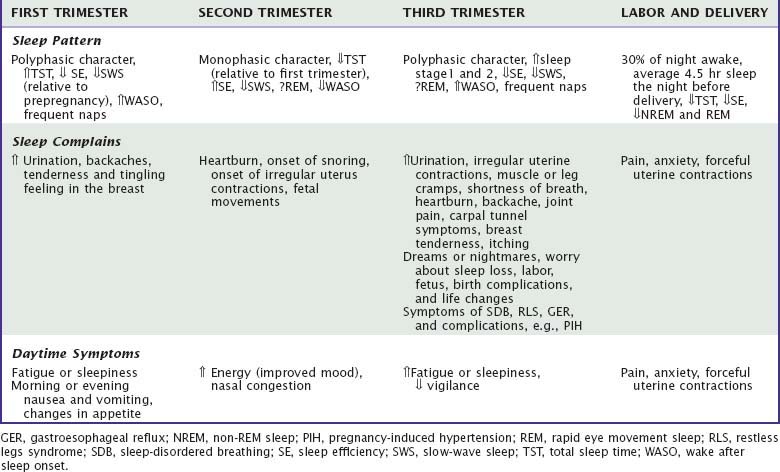
Sleep architecture, quality, and quantity are altered by pregnancy due to hormonal changes, physical discomforts, and psychological adjustments.8,9,28 Thus, the majority of women (66% to 97%) report sleep alterations, usually in the form of multiple nocturnal awakenings, becoming more obvious in the third trimester.1–327
Studies using objective (e.g., polysomnography [PSG] and actigraphy) and subjective sleep measures (e.g., sleep questionnaires) consistently report that sleep efficiency progressively decreases due to increased nocturnal awakenings after sleep onset.1,2,5,30,36–39 However, the findings from the objective sleep measures are inconsistent, likely because of limited sample sizes, different research methodology, and settings (laboratory or home). It appears that home-recorded PSG results in greater mean total sleep time (TST) and sleep efficiency and shorter sleep-onset latency and fewer minutes awake after sleep onset compared to laboratory-recorded PSG.40 Sleep during the daytime (naps), which can add to the total 24-hour sleep time, are often not recorded in PSG studies.3,5,39,41,42
First Trimester
Sleep starts to change around the 10th week of gestation, showing a polyphasic characteristic with both nighttime sleep and frequent daytime naps.5,36,39 A study using home PSG also revealed an average 34-minute increase in TST, but decreases in sleep efficiency and SWS during the first trimester compared with prepregnancy assessments.36 Another study also found an increased TST and frequent napping in the first trimester.5 Similarly, a survey study reported that the TST significantly increased from 7.8 hours in before pregnancy to 8.2 hours, but subjective sleep quality decreased.2
Second Trimester
Sleep becomes monophasic with improvements of subjective and objective sleep parameters, such as better sleep efficiency and less wake after sleep onset (WASO) (Table 138-1), but number of awakenings starts to increase at the end of the trimester.2,5,36,37,39,41 Objective measures of SWS and REM sleep stages are inconsistent.5,36–39 However, a study conducted in a home environment showed that SWS percentage slightly decreased but REM sleep percentage did not change relative to the first trimester.39
Third Trimester
In this trimester (see Table 138-1), sleep again shows a polyphasic character with more frequent or longer wake episodes after sleep onset (average, 2.6) and more naps. The mean night sleeping time is reported to be around 7.8 hours with decreased sleep efficiency.2,5,30,36–40,43,44
Women spent more time in sleep stages 1 or 2.5,30,39,45 Most PSG studies reported that SWS decreases when compared to age-matched control women or the same women in previous trimesters (13% to 20%).5,30,36,37,39,43 Spectral analysis of the EEG in NREM sleep also showed a progressive reduction of power density across pregnancy. The largest decrease (30%) occurred in the 14.25- to 15.0-Hz band in the third trimester relative to the first trimester.37 However, a longitudinal study reported a progressive rise in SWS across pregnancy in five women from early to late pregnancy.38 REM sleep (represented either as an absolute number of minutes or as a percentage of TST) has been reported to either remain the same or decrease slightly.36–3843 REM sleep latency is also highly variable across a number of studies.30,36–39,43
Labor and Delivery
Pain, anxiety, uterine contractions and administration of medications all affect sleep during labor and delivery.5,33,46 The sleep pattern alters and sleep quality decreases as women approach labor and immediately after delivery.5,46 In a descriptive longitudinal study of 35 women, sleep quality deteriorated progressively over the last 5 days of pregnancy and was the lowest on the night before hospital admission.46 Most women experienced spontaneous labor onset with forceful contractions during the night, presumably as a result of the peak of oxytocin level.9,46 There was also a significant relationship between the amount of sleep the night before hospitalization and pain perception in women with spontaneous labor onset. Women who did not experience labor onset had similar patterns of sleep loss. Thus, anxiety in addition to other contributors to sleep disturbance on that night must be considered.46 In a qualitative study of 20 women, all reported that they were unable to sleep once contractions began. As the latent phase of labor becomes prolonged, sleep may become impossible even with sleep aids.33 This can also occur as a result of the concomitant increase in estrogen and decrease in progesterone.20
Parity
Differences in sleep characteristics during pregnancy are also influenced by parity (primiparous or multiparous) or the presence of other children living in the home. Studies found that TST, SWS, and REM sleep were not significantly changed by parity in small samples.36,47 However, multiparas had consistently more sleep time but significantly lower sleep efficiency compared to nulliparas during pregnancy due to frequent awakenings.36 This finding, however, was contradicted by a later study using actigraphy.41 Multiparas may be awakened by their child(ren) during the night across pregnancy.1,3 Therefore multiparas might compensate for their disrupted sleep by spending more time in bed. However, it has been noted that during the third trimester, younger women (<30 years) had more TST than older women (>30 years), whose sleep may have been affected by parity or other children’s needs.2
In another study, while sleep efficiency of nulliparas decreased from 90% (third trimester) to 77% (1 month after delivery), sleep efficiency for multiparas did not change significantly (87% in third trimester, 84% 1 month after delivery).47
Energy levels are affected by parity such that nulliparas experienced higher energy levels than did multiparas at all times during pregnancy.12 This suggests that multiparas may be older, have difficulty taking a nap, and have insufficient nocturnal sleep due to other children in the home.3,29 Thus, parity should be controlled in sleep research with pregnant women.
Sleep-Related Disorders in Pregnancy
Sleep-Disordered Breathing
Sleep-disordered breathing refers to the entire spectrum of breathing disorders during sleep, ranging from intermittent snoring without apnea to the most severe form of SDB, arguably the obesity-hypoventilation syndrome.48 Nighttime features of SDB include snoring, shortness of breath (dyspnea), breathing pauses, and sudden awakenings with choking sensations. Snoring and shortness of breath are common during pregnancy, starting at the end of second trimester. As pregnancy progresses, obstructive sleep apnea (OSA) can also develop, especially in obese women.6,25,43,49
SDB can be attributed to changes in the respiratory system (upper airway, lung mechanics, and control of breathing) during pregnancy. Although some changes cause SDB, others actually protect from it. Potential risk factors for SDB are detailed in Figure 138-1 and include a reduction in upper airway size (due to weight gain, increased fluid volume, nasal congestion etc.), reduced functional residual capacity and residual volume, increased minute ventilation, supine position, and fragmented sleep.4,8,18,34,43,45,48,50–52 The temporal relationship between fragmented sleep and the pathogenesis of SDB in pregnancy requires further study. Active and passive smoking can also contribute to loud snoring and breathing problems during sleep.53
The low incidence of SDB in pregnant women likely results from physiologic changes that can protect them from SDB. High circulating progesterone during pregnancy can protect the upper airway from obstruction, increasing upper airway dilator muscle (genioglossal) activity and its responsiveness to chemical stimuli (CO2) during sleep.48 A right-shifted oxyhemoglobin dissociation curve and increases in heart rate, stroke volume, and cardiac output with reductions in peripheral vascular resistance improve the delivery of oxygen to the placenta and maternal tissues.8,25,48 Also, as pregnancy advances, less time is spent in the supine position during sleep.30,43 This decreases the possibility of adverse respiratory events43,45,48 and of supine hypertensive syndrome.42
Numerous survey studies report that both the severity and frequency of snoring increase steadily during pregnancy.2–4,26,35,54–56,57 However, the prevalence of snoring is variable, ranging from 10.4% to 46% in the third trimester, likely owing to different study designs, reliance on self-reported snoring rather than asking her bed partner, or asking about habitual snoring rather than both habitual and occasional snoring.26,34,35,59
The precise prevalence of OSA during pregnancy is also unknown. Large prospective studies using objective methods are lacking. In one study of 267 women, none had an apnea–hypopnea index (AHI) of 5 or more in their second trimester, but habitual snorers spent between 61% and 92% of TST with snoring.26 Thirteen of these snorers had abnormal breathing patterns (sustained effort and crescendos) compared to nonsnorers. It has been estimated that more than 10% of pregnant women may be at risk of developing OSA during pregnancy.55 Pregnant women with apnea symptoms have a higher likelihood of gestational hypertensive disorders, gestational diabetes, and unplanned Caesarian sections.55a
Normal pregnant women have been reported to exhibit modest oxygen desaturations during sleep, which would be worse in the supine position.26,30,45,58 The mean overnight oxygen saturation (SaO2) in nonpregnant women (98.5%) was significantly higher than the 95% seen in normotensive pregnant women or women with pregnancy-induced hypertension.60 Small PSG studies of healthy pregnant women with or without multiple-gestation pregnancy showed that none had clinically significant OSA.43,53,59 However, case reports and studies of pregnant women with OSA indicate that those with obesity, large neck circumference, abnormal oropharyngeal anatomy, or a small oropharynx are at risk for developing OSA during pregnancy or developing a more severe case of OSA if it is diagnosed before pregnancy.6,43,48,60 However, AHI significantly improves after delivery.6
Diagnosis
SDB associated with adverse fetal and maternal outcomes is mentioned later. Because of such outcomes, early diagnosis and treatment of SDB, especially OSA, is important. SDB is likely underdiagnosed during pregnancy because daytime sleepiness is considered a normal part of pregnancy or because women are unwilling to complain about snorting, gasping, or snoring.14 Thus, women without prepregnancy SDB who have predisposing factors require careful surveillance, because SDB can develop with pregnancy progression.4,6,43,55,61 Pregnant women with prepregnancy SDB might also need to be reassessed, particularly after the sixth month of pregnancy, because symptoms can reoccur or worsen with nasal congestion and weight gain.6,63
Sleepiness in pregnant women is not specific to SDB.35 Studies show that snoring pregnant women and preeclamptic women with inspiratory flow limitations (IFLs) do not necessarily demonstrate sleepiness as measured clinically by the Epworth Sleepiness Scale.35,42,57 This may be due to their regular daytime napping or the fact that they describe their sleepiness with words like “unrefreshed,” “fatigue,” or “tired.” Scales measuring fatigue might give more information about sleepiness. Thus, in the clinical setting, questions about SDB should extend beyond simply asking about excessive daytime sleepiness. Objective measures (e.g., multiple sleep latency test (MSLT)) are needed to determine the sleepiness level of women with normal and complicated pregnancies.
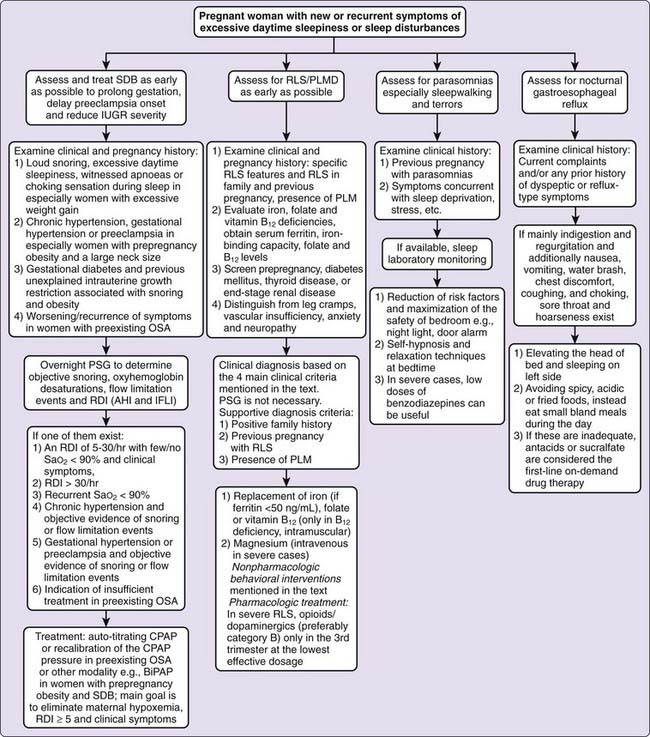
Women with chronic or gestational hypertension, preeclampsia, gestational diabetes, or a history of infants with unexplained intrauterine growth restriction should be closely evaluated for SDB, especially if they have predisposing factors such as obesity.7,14,42,48,60,62 Women with any of several different problems (Fig. 138-2) should undergo an overnight PSG (with both nasal cannula pressure transducer and thermistor) to measure oxyhemoglobin desaturations, RDI or AHI and IFL index (IFLI).6,7,14,42,48,60,61,62,63 If IFLI is not measured during sleep, abnormal respiratory events would be missed, especially for preeclamptic women, because most of them experience nonapneic flow limitations during sleep associated with blood pressure surges.7,63
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree