Chapter 128 Sleep in Chronic Kidney Disease
Abstract
Chronic kidney disease (CKD) has been defined by either a chronic decrease in kidney function or other evidence of chronic kidney damage such as proteinuria or cystic disease.1 The prevalence of CKD is increasing,2 and in view of the growing prevalence of the metabolic syndrome, diabetes, and hypertension, this trend is expected to continue.3 As a result, CKD is a burgeoning public health problem that has been strongly linked to an increased risk of sleep disorders. It is estimated that there are 15 million adults in the United States with stage 3 or greater CKD (defined by an estimated glomerular filtration rate [eGFR] <60 mL/min/1.73 m2).2 An additional 10 million have CKD of lesser severity, with an eGFR between 60 and 90 mL/min/1.73 m2 or microalbuminuria, an early indicator of kidney damage, as well as an independent risk factor for cardiovascular events.4,5
Definition of Terms
The various terms and treatments used in caring for patients with kidney disease may be unfamiliar to sleep clinicians and researchers. Therefore Box 128-1 provides a descriptive list of the terms and dialysis-management strategies discussed in this chapter for concise, albeit simplistic, descriptive reference.
Box 128-1 Definitions of Terms and Dialysis Strategies in Chronic Kidney Disease
Kidney Disease
The term chronic kidney disease encompasses all degrees of kidney dysfunction.6 At one end of the spectrum is mild kidney disease, which is very common, and although it has been associated with poor outcomes, it may present few bothersome symptoms to patients and is largely underrecognized. At the other end of the CKD spectrum, end-stage renal disease (ESRD) is defined as kidney failure for which medical management alone is inadequate and is usually associated with a glomerular filtration rate less than 15 mL/min/1.73 m2. ESRD results in the inability to excrete waste products, control serum electrolytes, handle the daily dietary and metabolic acid load, and maintain fluid balance. The treatment options for ESRD are listed in Box 128-1.
Treatment
Hemodialysis passes the patient’s blood through a semipermeable membrane by which toxins and fluid are removed by diffusion and convection. Traditionally, patients undergo hemodialysis thrice weekly at a dialysis center during morning, afternoon, or evening shifts. In peritoneal dialysis, a catheter is inserted into the peritoneum through which fluid (dialysate) is infused and then drained after a suitable dwell time. The peritoneal membrane functions as a semipermeable filter facilitating the removal of waste products and fluid. Continuous ambulatory peritoneal dialysis (CAPD) describes the strategy in which the patient or caregiver instills fluid into the abdomen and then drains it after a dwell time of 4 to 6 hours during the day. Instillation and subsequent drainage of fluid after a period of dwell time is termed an exchange. The CAPD patient also usually has a nighttime dwell of peritoneal fluid, and the fluid is drained on awakening. In nocturnal peritoneal dialysis (NPD), the fill and drain exchange process is automated and is performed at night while the patient is asleep. Nocturnal hemodialysis is another contemporary approach whereby ESRD patients undergo hemodialysis 3 to 7 nights a week as well as short daily hemodialysis in which patients do hemodialysis 6 days a week for 2 to 2.5 hours.7 Kidney transplantation and palliative care are additional management strategies.
Epidemiology
Symptoms related to sleep and fatigue are common, persistent, and important to patients with advanced CKD and ESRD.8–15 In a systematic review of 59 studies to estimate the prevalence of symptoms among patients on dialysis, several of the most common and severe were related to sleep and fatigue, with fatigue or tiredness endorsed by an average of 71%, disturbed sleep by 44%, and restless legs by 30%.16 Similar to those on dialysis, patients with advanced CKD have been shown to have a pattern of sleep and fatigue symptoms that are only slightly less severe than persons with ESRD.17 The need to identify and manage patients in this population who have complaints regarding sleep quality is underscored by a high rate of use of hypnotics.12,18 Studies reporting use of hypnotics likely underestimate the prevalence because patients also use diphenhydramine, antidepressants, and opiates to promote sleep.
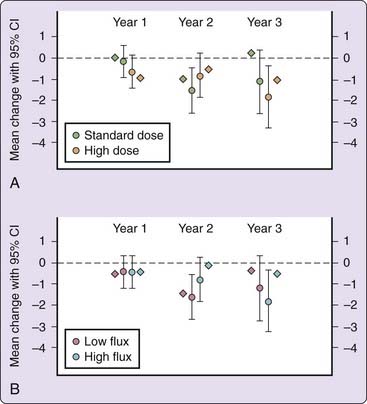
Subjective sleep quality is also clinically meaningful for CKD patients because sleep problems may be an indication to initiate or modify dialysis management. For example, nephrologists increase the dose of dialysis in an effort to mitigate symptoms related to sleep problems. This practice was tested as a prespecified quality-of-life outcome in the Hemodialysis (HEMO) Study, a multicenter study of hemodialysis dose and membrane flux.19 The dose of dialysis reflects the adequacy of small molecule clearance, and high flux increases the clearance of middle molecules such as β2-microglobulin and parathyroid hormone. As shown in Figure 128-1, there was no significant effect of high-dose dialysis on sleep quality. High-flux hemodialysis had a small beneficial effect on sleep quality that did not reach significance after accounting for multiple comparisons made by the HEMO Study investigators.19 Despite receiving an adequate dose of hemodialysis, longitudinal analysis of the HEMO Study demonstrated that approximately 30% of study participants experienced a clinically significant decline in sleep quality over the course of 3 years.20
In the CKD and ESRD population, complaints of sleep problems have been linked to disability days, use of health care, and health-related quality of life.21–24 Also, both dialysis and kidney transplant patients with decreased sleep quality have a significantly higher mortality rate than similar patients with good sleep.23,25,26 Attribution of causality is difficult because most information in this regard comes from observational studies, but the poor quality of life and increased risk of death among those with worse sleep quality might reflect acquired sleep disorders, the negative impact of poor sleep quality on mood, or even the treatment of sleep problems. Nonetheless, symptoms related to sleep and fatigue are common and persistent, and patients on dialysis strongly value improvement in these symptoms.27 In a study of 100 hemodialysis patients, 93% would favor more frequent hemodialysis treatments if it improved fatigue, and 57% would do more frequent treatments to improve sleep.27 This contrasts with 19% of patients who are willing to undergo more frequent dialysis to extend their life up to 3 more years.27 These findings underscore the profound impact of sleep on the experiences of patients with CKD and ESRD.
Subjectively and Objectively Assessed Excessive Daytime Sleepiness in Patients with End-Stage Renal Disease
Excessive daytime sleepiness (EDS) is highly prevalent in patients with ESRD. Assessments using standardized questionnaires demonstrate that 52% to 67% of ESRD patients endorse EDS.28,29 Moreover, EDS has been demonstrated in both hemodialysis and peritoneal dialysis patients using the multiple sleep latency test (MSLT).30–32 The average sleep latency was 6.3 minutes by MSLT among patients undergoing CAPD.30 Hanly and colleagues reported that the subjects with shorter sleep latency had a higher BUN (blood urea nitrogen, reflecting a greater uremic burden) and significantly more-frequent periodic limb movements (PLMs). Although there was a direct association between severity of uremia and sleep latency, there was not a significant improvement in daytime sleepiness after conversion from conventional hemodialysis to nocturnal hemodialysis.31
Daytime sleepiness can contribute to the poor vocational and rehabilitation potential traditionally associated with ESRD.33 In view of the notably high prevalence of EDS in patients with ESRD and the multiplicity of potential etiologies, a comprehensive history, physical examination, and referral to a sleep specialist may be a first step in the evaluation of daytime sleepiness in patients with CKD.
Insomnia in Patients with Chronic Kidney Disease
Insomnia is common and has substantial health consequences in patients with kidney disease. Insomnia reflects difficulty falling asleep, maintaining sleep, or waking early in the morning, with associated daytime difficulties (see Chapter 78). Although the prevalence of poor sleep quality of patients with CKD has been shown to be high,34,34a the majority of studies have examined patients undergoing dialysis. Insomnia among dialysis patients has been associated with decreased quality of life, and a worsening of insomnia over the first year of dialysis has been associated with an increased mortality risk.23
Up to two thirds of dialysis patients experience insomnia,13–15,35,36 although most of the studies have been limited to examining the prevalence of symptoms of poor sleep initiation and maintenance. These subjective complaints are consistent with short and fragmented sleep as measured by polysomnography and wrist actigraphy in these patients. A comparison of 46 conventional hemodialysis patients to 137 controls from the Sleep Heart Health Study (SHHS) demonstrated a higher prevalence of short sleep (<5 hours) (odds ratio [OR], 3.27; 95% confidence interval [CI], 1.16 to 9.25) and decreased sleep efficiency (OR, 5.5; 95% CI, 1.5 to 19.6).37 This study also demonstrated a higher subjective burden of insomnia, with hemodialysis patients reporting more difficulty getting back to sleep (OR, 2.25; 95% CI 1.11 to 4.60) and awakening too early (OR, 2.39; 95% CI, 1.01 to 5.66).
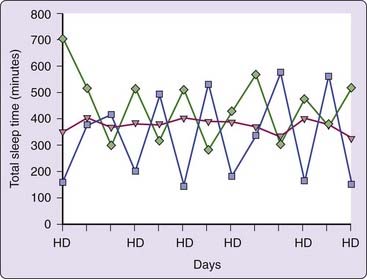
Wrist actigraphy has revealed notable variability in sleep–wake pattern among patients undergoing hemodialysis as shown in Figure 128-2. In this study, those with early-morning hemodialysis had significantly shorter nocturnal sleep time compared with those who had later hemodialysis shifts when averaged over the course of 2 weeks. In addition, those with increased variability in sleep times were found to have higher levels of daytime sleepiness.38 Because kidney transplantation reduces the need to attend a fixed dialysis schedule, insomnia associated with ESRD can improve after kidney transplantation. Using the Athens Insomnia Scale, the prevalence of chronic insomnia after kidney transplantation was 8% compared to 15% among wait-listed patients on hemodialysis.13
Aside from addressing underlying disorders known to cause insomnia, insomnia treatment among patients with kidney disease is guided by limited evidence. Caution should be exercised with regard to pharmacologic intervention in this population because some hypnotics such as diazepam and some nonhypnotics such as gabapentin used by patients to promote sleep require changes in dosing with underlying renal disease. There are also potential drug-drug interactions in this population in which polypharmacy is common, and there have been only a few small trials of insomnia treatment. A small study of zaleplon in the hemodialysis population showed improved sleep efficacy.39 Twenty-four peritoneal dialysis patients with insomnia without active psychiatric illness were randomized to receive cognitive behavioral therapy (CBT) and counseling regarding good sleep hygiene or just sleep hygiene counseling.40 After 4 weeks, there were substantial improvements in the Pittsburgh Sleep Quality Index (PSQI) and the Fatigue Severity Scale in the CBT group.40 This demonstrates that patients undergoing peritoneal dialysis might benefit from CBT, and the use of this treatment should be considered in light of the potential problems associated with the polypharmacy.
One approach to the management of insomnia among patients with CKD consists of providing counseling to optimize sleep hygiene, screening for other sleep disorders such as sleep apnea and restless legs syndrome (RLS), providing a brief trial of cognitive behavioral therapy and hypnotics, and considering an overnight PSG in patients who remain symptomatic. For patients undergoing hemodialysis, it may be reasonable to ask the patient to avoid napping during dialysis sessions and to consider using cool dialysate for hemodialysis. In this regard, ongoing work is examining the influence of dialysate temperature on the circadian rhythm of patients undergoing thrice-weekly hemodialysis. This work is based on the concept that standard hemodialysis disrupts circadian rhythms by delivering a heat load with the combination of volume removal, which increases core temperature, and warm dialysis (37° C), which is usually a higher temperature than the core temperature of the hemodialysis patient at the time of treatment.41 Parker and colleagues demonstrated that patients receiving hemodialysis are subject to a heat load and have proposed that cool dialysate (35° C) hemodialysis might ameliorate the sleep problems in this population.42 Patients using overnight peritoneal dialysis might need to adapt their regimen to avoid frequent alarms and the sensation of nocturnal abdominal discomfort due to the automated filling and draining of dialysate.
The use of melatonin in the ESRD population has received only limited evaluation. Studies have demonstrated both increased and depressed daytime melatonin levels among patients undergoing hemodialysis.43,43a Despite these conflicting findings, the use of melatonin for treatment of insomnia in ESRD merits further research44 because investigators have consistently demonstrated attenuation of the nocturnal surge of melatonin among both humans and animal models of ESRD. Pilot testing in ESRD patients demonstrated a substantial beneficial effect of melatonin on sleep in ESRD patients.45
Restless Legs Syndrome in Patients with Chronic Kidney Disease
Epidemiology
End-stage renal disease has been strongly associated with RLS. A comprehensive discussion of RLS and PLMD is provided elsewhere in this volume (see Chapter 90). The majority of investigations examining RLS in CKD patients have been cross-sectional studies of chronic dialysis patients, thereby limiting our understanding of the natural history of RLS in the broader CKD population as well as how it relates to the progression of kidney disease. A study has outlined the difficulties of using questionnaires to diagnose RLS in the ESRD population, but work operationalizing the International Restless Legs Syndrome Study Group (IRLSSG) definition of RLS has demonstrated a relatively consistent estimate of RLS ranging between 20% and 30%.46 Studies using a clinical assessment have found that approximately 23% to 33% of chronic hemodialysis patients have RLS, and data from our center have also shown a 30% prevalence of RLS among hemodialysis patients using a questionnaire based on IRLSSG criteria.47
Etiology and Risk Factors
Although the estimates of RLS among the hemodialysis population range up to 10 times more common than in the general population,48 the etiology and risk factors for RLS in those with ESRD remain unclear. Because uremic RLS is improved with dopamine agonists, opioids, and gabapentin, it seems likely that the pathophysiology is similar to that of idiopathic RLS. It may be that patients with ESRD are primed for RLS by increased neural excitability secondary to uremic neuropathy. Furthermore, patients undergoing hemodialysis are challenged by their treatment regimen, which mandate periods of relative immobility for up to 4 hours thrice weekly, perhaps unmasking subclinical RLS. In the general population, it has been demonstrated that RLS is caused by blockade of the D2 receptor in the diencephalon.49 However, small studies in ESRD have correlated RLS to a number of factors, including iron deficiency, calcium levels, calcium channel blockers, parathyroid hormone levels, hemoglobin, dialysis timing, and dialysis adequacy.
Morbidity and Mortality
RLS has been associated with substantial morbidity and mortality in the ESRD population. As in the general population, RLS has been associated with poor mental health in patients receiving hemodialysis. Symptoms of restless legs were associated with a lower health-related quality of life (HRQOL) among a nationwide sample of 900 dialysis patients.50 A causal link between insomnia and RLS in the ESRD population has not been established using treatment trials; however, RLS was associated with increased risk of insomnia in a sample of 333 hemodialysis patients.51
RLS has also been associated with shorter survival in hemodialysis patients after controlling for age, sex, and duration of dialysis.50,52 In patients with RLS, the inability to sit still can lead to shortening treatment times or skipping hemodialysis treatments. RLS was associated with hemodialysis nonadherence and it may be that poor adherence to the dialysis prescription, rather than the RLS per se, contributes to the increased mortality risk.52 It has also been demonstrated that RLS and PLMD increase sleep fragmentation, and this fragmented sleep can in turn promote hypertension.53 Lastly, it may be that RLS severity is a marker for inadequate dialysis, inflammation, or PLMs. RLS remains a major diagnostic and therapeutic challenge for those caring for patients undergoing dialysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree