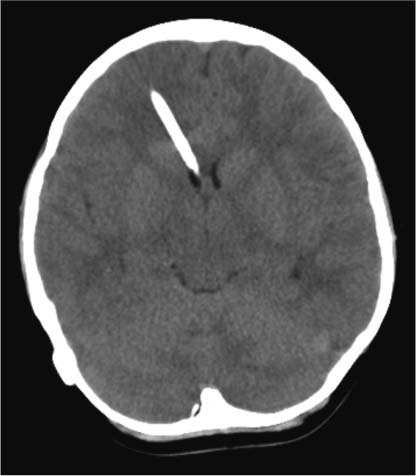
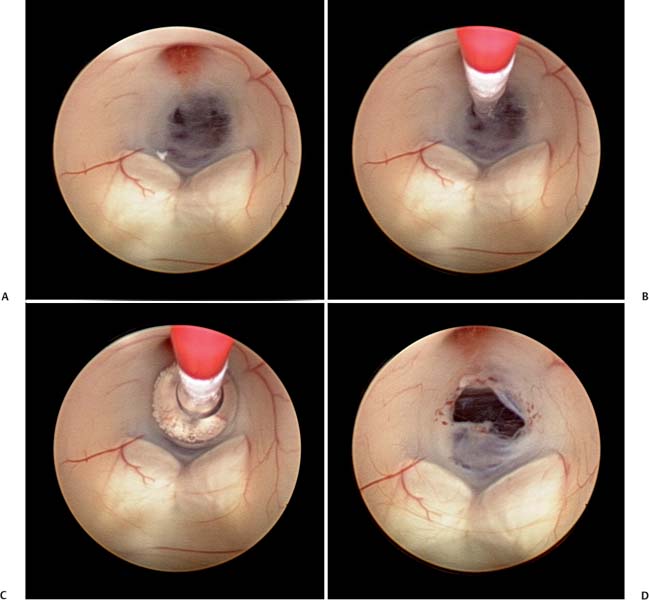
5 Slit Ventricle Syndrome The development of the ventriculoperitoneal (VP) shunt has dramatically improved the prognosis for patients with hydrocephalus, but it has produced problems of its own. Slit ventricle syndrome (SVS) represents one of the more difficult shunt-related diseases to treat largely because there is no consensus and little evidence as to what exactly it is, why it occurs, and how best to treat it. Even the nomenclature is confusing: the syndrome has been variously named normal volume hydrocephalus,1,2 noncompliant ventricle syndrome,3,4 shunt-related pseudotumor cerebri,5and small ventricle–induced cerebrospinal fluid (CSF) shunt dysfunction.6 Some have questioned whether the syndrome even exists at all.7 Although small “slitlike” ventricles are a frequent occur-rence in long-standing shunted hydrocephalus, it does appear that there is a subset of shunted patients who have symptoms of shunt dysfunction with elevated intracranial pressure (ICP) and no evidence of ventricular dilation. The formal definition of SVS involves the clinical triad of intermittent transient symptoms of shunt dysfunction (defined as headaches lasting 10–90 minutes), small ventricles on computed tomography (CT), and slow shunt valve refill.8This condition has been estimated to affect between 6 and 22% of all patients who have radiologic slit ventricles and headaches.4,9 In one series of 370 shunted patients, 11.5% developed SVS, and 6.5% required surgical intervention, despite the finding of radiographic slit ventricles in > 60%.10 SVS does not present during infancy11 or when hydro-cephalus occurs in adults.2 Most commonly, it presents between 5 and 10 years of age in patients who had been shunted during infancy (Fig. 5.1).11,12 Because headache is common in shunt patients, SVS must be differentiated from other conditions that can cause headaches in patients with radiologic slit ventricles, such as CSF overdrainage, intermittent proximal shunt dysfunction, and migraine.4,13 One study used invasive ICP monitoring to differentiate five subtypes of “slit ventricle syndromes”: low-pressure headaches due to overdrainage, intermittent proximal shunt obstruction, true shunt failure without ventriculomegaly, intracranial hypertension with a working shunt, and headaches unrelated to shunt function.2 In SVS, the ventricles do not enlarge in response to elevated ICP produced by shunt malfunction. Intermittent symptoms are presumed to be caused by catheter obstruction produced by small ventricles that leads to increased pressure, marginal dilation, and restoration of catheter function.2,4,9 Patients with SVS have noncompliant ventricles that remain very small even in the face of markedly elevated ICP. Three theories have been advanced as to why this is the case, and it is possible that more than one or even all three are correct. Fig. 5.1 Computed tomography scan of a 10-year-old girl who had been shunted during infancy. Despite small ventricles, lumbar puncture demonstrated high opening pressure, and a proximal shunt malfunction was found during surgical exploration. The first theory to explain noncompliant ventricles is that there is increased ventricular wall stiffness in SVS that prevents expansion in response to pressure changes. This is thought to be due to chronic changes in the ventricular wall, such as subependymal gliosis in patients with long-term ventricular drainage. Direct measurement of ventricular compliance demonstrated increased ventricular elastance in four SVS patients.1 A canine model of long-standing shunted hydrocephalus with overdrainage has shown histo-logic evidence of subependymal and periventricular gliosis that presumably led to increased elastance,14 although these findings were not confirmed in autopsy examination of one patient with small ventricles and a long-standing ventricular shunt.15 A second proposed mechanism for noncompliant ventricles in SVS argues that overshunting of infantile hydrocephalus leads to craniocerebral disproportion with microcephaly and synostosis that produces very small ventricles that are unable to expand. Dampening of cerebral pressure waves might cause understimulation of growth of the calvaria and premature ossification of sutures.16,17 It has long been known that chronic shunting produces dramatic changes in the skull, especially thickness,18 and one study of 400 patients shunted in infancy found overdrainage associated with microcephaly and synostosis in > 8%.19 Microcephaly does occur in SVS,12 histologic evidence of craniosynostosis has been observed,16 and subtemporal decompression and other cranial expansion techniques have been used with some therapeutic success.9,17 A third theory about etiology of SVS postulates that the problem is with venous hypertension leading to poor absorption of CSF at the level of the arachnoid granulations. It is known that CSF is not absorbed until ICP is 3 to 6 mm Hg higher than pressure in the venous sinuses, particularly the superior sagittal sinus.20 High venous pressure can lead to hydrocephalus in achondroplasia.21,22 Venous bypass has been used successfully to treat selected cases of hydro-cephalus,23 and other intracranial hypertension syndromes (particularly pseudotumor cerebri) are believed to occur because of elevated venous pressure.20 Chronic shunting of hydrocephalus in patients with distensible heads could lead to uncoupling of ventricular and venous pressure due to dampening of the normal intraventricular pulse pressure. High venous pressure in SVS patients could then prevent appropriate absorption of CSF, leading to distention of cortical subarachnoid spaces as well as increased cerebral elastance due to venous congestion.9,20 SVS patients may therefore have an acquired form of shunt-related pseudotumor cerebri.5 A significant number of SVS patients will improve without surgical intervention.24 Conservative treatment options include observation, hydration, diuresis, and corticosteroid treatment. Antimigraine therapy has been successfully used to treat SVS patients, possibly by reducing venous congestion or improving coupling of CSF absorption to ICP.25,26 It is unclear whether patients who improve without surgical intervention truly have SVS or simply small ventricles with shunt-associated headaches that are not related to ICP.5 In the setting of slit ventricles and low-pressure headaches, it is possible that the problem involves simple CSF overdrainage. In this case, increasing the valve pressure or adding an antisiphon device can be very effective.8 Clinical improvement is not always accompanied by reexpansion of the ventricles.8 Some patients with overdrainage syndromes in fact will not require a shunt at all. One trial of externalization with ICP monitoring in 22 patients with SVS demonstrated that 5 did not require shunting and underwent removal of the shunt without further treatment.27 Increasing intracranial volume could be an effective treatment for SVS by reducing the effects of decreased compliance, especially if synostosis and microcephaly are present. Subtemporal decompression was first performed by Cushing for raised ICP and later by Dandy to treat pseudotumor cerebri and was popularized as a treatment for intermittent shunt obstruction due to small ventricles by Epstein et al.28 The procedure involves removal of part of the temporal bone to create an artificial fontanelle. This allows release of pressure and can vent pressure waves that presumably occur as a result of abnormal buffering capacity.3 One study of 22 SVS patients treated with ipsilateral subtemporal decompression with the dura left open found that shunt-related admissions were decreased by 75% and shunt revisions by nearly 80%.29 Subtemporal decompression by itself can dramatically reduce ICP6,30 and seems to work even if the ventricle does not enlarge postoperatively.31 In a study of 32 SVS patients, subtemporal decompression was associated with an early increase in the number of shunt revisions in the early postoperative period, but the number of admissions for raised ICP was reduced.3 Other more extensive calvarial expansion techniques have also been used to treat SVS based on the observation that widespread pathologic suture fusion occurs in this condition. In one report, 14 patients with intracranial hyper-tension in spite of a functioning shunt were treated with craniotomy and morcellation of bone from the coronal suture to the transverse sinus and to the squamosal sutures on either side, and symptoms were improved in all patients.9 Another study involved three patients treated with a biparietal craniotomy with reorientation of the bone flap to provide more intracranial volume. In this study, ICP was monitored invasively before and after the operation and was observed to be dramatically improved in each patient.17 In a study of five SVS patients treated with cranial expansion and removal of sclerotic sutures, all were asymptomatic at 24 months postoperatively.16 Lumboperitoneal (LP) shunting is postulated to be an effective treatment for SVS because it allows for drainage of the cortical subarachnoid space as well as the ventricles.5,32,33 LP shunts are often avoided in the pediatric population due to fear of scoliosis, pain, neurologic changes, and hindbrain herniation.34 However, if valves are used, and LP shunts are placed only in older children, these complications are rare.5Reestablishment of a pressure gradient from the ventricles to the subarachnoid space allows normalization of ventricular size, and subarachnoid drainage prevents distention of cortical subarachnoid spaces. In one early report, a patient with SVS and progressive shunt dysfunction was confirmed to have communicating hydrocephalus by cisternogram, then underwent placement of an LP shunt that alleviated his symptoms.35 A more recent series of LP shunt insertion showed clinical improvement in seven SVS patients.32 Each patient had symptoms of intermittent shunt malfunction and a functioning shunt demonstrated by shunt tap or surgical exploration, and the VP shunts were not removed. In three of these patients who now had VP and LP shunts, subsequent VP shunt dysfunction actually led to ventriculomegaly,36 supporting the hypothesis that LP shunting allows a pressure gradient from the ventricles to the subarachnoid space. If communication of CSF can be demonstrated using radiographic techniques, LP shunting can be used by itself with permanent removal of the VP shunt.33 In a series of 27 patients with severe SVS, incapacitating headaches, and recurrent proximal shunt malfunction, CT scan after intraventricular injection of iohexol verified communicating hydrocephalus in 24 patients. These patients underwent VP shunt removal and placement of an LP shunt with resolution of symptoms and normalization of ventricular size. There were no cases of hindbrain herniation or other complications.33 Another series of 33 patients with SVS possibly due to an isolated ventricle underwent replacement of the VP shunt with an LP shunt. Postoperatively, all patients had resolution of symptoms, there were fewer subsequent shunt revisions and infections, and no patient experienced hind-brain herniation.7 For patients with lumbar anatomy that precludes LP shunt placement, cisterna magna-ventricle-peritoneum shunts have been used with good results.37 Endoscopic third ventriculostomy (ETV) is an appealing treatment for hydrocephalus because patients can become shunt-free even after prolonged shunt reliance (Fig. 5.2).38 ETV for SVS seems counterintuitive, as ventricular enlargement is one of the principal requirements for safe ventriculoscopy. As a result, early attempts were mainly performed as a last resort in patients for whom other treatments had failed. In one study, five patients with aqueductal stenosis underwent ETV after failure of valve upgrade and subtemporal decompression, and all five had encouraging results.39 In another series including seven SVS patients undergoing ETV, two were rendered shunt-free, and the other five had improvement of symptoms.10 More recently, several techniques have been used to produce ventricular enlargement in preparation for ETV, such as externalization of the shunt with40 or without27 controlled intracranial hypertension, or placement of a programmable valve41,42 or high-pressure antisiphon device.43In one study of 22 patients with SVS, patients underwent shunt externalization and invasive ICP monitoring, and all 16 patients who demonstrated a need for continued shunting underwent ETV regardless of the putative cause of hydrocephalus.27 Ten of these patients became shunt-free. Of the six failures, four were apparent prior to discharge from the hospital, and the other two had scarring over the ventriculostomy defect. In a series of four patients with SVS and small ventricles, VP shunts were externalized, and the external ventricular drainage bag was gradually elevated to an average of 18.8 cm above head level over an average of 5.8 days to render the ventricles navigable. Three of the four patients were successfully rendered shunt-free.40 In another study, 15 patients with SVS underwent ventricular cannulation using a very small flexible endoscope to measure compliance.42 In 4 patients, compliance was low, and ETV was performed using a larger endoscope; in the other 11 patients, a shunt with a programmable valve was inserted and the pressure slowly increased over an average of 16.3 months, after which ETV was performed. All 15 patients in this study became shunt-free with no symptoms. ETV for SVS is not without risks, as these patients frequently have adhesions that make ventricular cannulation and navigation difficult and potentially hazardous. Additionally, two studies have identified transient short-term memory loss as a risk of ETV in SVS patients.27,39 Any attempt to formulate a treatment plan for patients with SVS should include a careful assessment of symptoms and measurement of ICP by means of intraparenchymal fiberoptic monitor placement or lumbar puncture. Conservative treatment is a good first step for any patient who does not have disabling symptoms. If a patient presents with symptoms of low-pressure headaches and low pressure on monitoring, valve upgrade can often be helpful. Evidence of intermittent shunt malfunction can usually be effectively treated by replacing the shunt in a different location, using stereo-tactic guidance if necessary. Subtemporal decompression is reserved for patients who have not responded to other treatments. Cranial expansion surgery may be effective in cases of synostosis. ETV is difficult in patients with SVS, and our general technique is to externalize the shunt and occlude it transiently with ICP monitoring in an attempt to enlarge the ventricles. Frameless stereotactic image guidance is useful in cannulating small ventricles with the endoscope. We perform LP shunting infrequently in the pediatric population. Fig. 5.2 Intraoperative photographs showing the steps of endoscopic third ventriculostomy in a patient with ventriculomegaly. (A) The thinned floor of the third ventricle is visible between the infundibular recess and the mammillary bodies. (B) A no. 4 French Fogarty balloon catheter is used to perforate the floor of the third ventricle, (C) then inflated to produce (D) an opening. This operation may be difficult to perform if the ventricles are small and do not enlarge with controlled shunt occlusion. SVS is a difficult entity to treat because of uncertainty regarding both the etiology and the most effective treatment for this condition. The principal theories as to the etiology of SVS involve decreased brain compliance, cephalocranial disproportion, and venous hypertension. Several treatments have been used successfully, including ventricular shunt revision, cranial expansion techniques, LP shunting, and ETV. With patience and careful application of these approaches, most SVS patients can be managed successfully with a satisfactory long-term outcome. The term slit ventricle syndrome is a catch-all term that refers to a severe headache disorder in patients with ventricular shunts and chronic headaches. Our original definition of the term defined the condition as a triad of small ventricles on imaging studies, severe headaches lasting 10 to 90 minutes, and slow or no refill of a shunt-flushing device.44 Many reports of the syndrome followed, but there was no consensus about its definition or evidence about the cause of the headaches. It was assumed that SVS was related to chronic overdrainage of CSF by the shunt system and that the resulting long-term changes in the brain or skull led to the headaches.45–47 Based on a retrospective review of patients with chronic headaches and shunts, we defined five distinct pathophysiologies that had been incorporated into the concept of SVS,48 including intermittent proximal obstruction; severely low ICP analogous to spinal headaches; cephalocranial dis-proportion, which occurs only in the context of genetic craniofacial syndromes (i.e., the patient has increased ICP despite a working shunt); and intracranial hypertension associated with nonresponsive ventricles and shunt failure. The latter has been called normal volume hydrocephalus (NVH) by Engel and colleagues, who originally described it.49
Surgical Management
Etiology
Treatment Options
Conservative Treatment
Shunt Revision/Removal
Cranial Expansion Procedures
Lumboperitoneal Shunt
Endoscopic Third Ventriculostomy
Management Plan
Conclusion
Lumboperitoneal Shunting
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree